Abstract
Membrane traffic requires vesicles to fuse with a specific target, and SNARE proteins and Rab/Ypt GTPases contribute to this specificity. In the yeast Saccharomyces cerevisae, the Rab/Ypt GTPase Ypt6p is required for fusion of endosome-derived vesicles with the late Golgi. We have shown previously that activation of Ypt6p depends on its exchange factor, Ric1p–Rgp1p, a peripheral membrane protein complex restricted to the Golgi. We show here that a conserved trimeric protein complex, VFT (Vps52/53/54), binds directly to Ypt6p:GTP. Localization of VFT to the Golgi requires Ypt6p, but is unaffected in gos1 and tlg1 mutants, in which late Golgi integral membrane proteins, including SNAREs, are mislocalized. The VFT complex also binds directly to the N-terminal domain of the SNARE Tlg1p, both in vitro and in vivo, in a Ypt6p-independent manner. We suggest that the VFT complex links vesicles containing Tlg1p to their target, which is defined by the local activation of Ypt6p.
Keywords: Golgi/membrane fusion/Rab GTPase/SNAREs/VFT complex
Introduction
A central problem in the organization of the membranes of the secretory pathway is how a vesicle derived from one compartment finds and fuses with its correct target. Key molecules involved in the fusion process are the integral membrane proteins called SNAREs, and the specificity with which SNAREs on vesicles and target membranes form four-helix bundles in part determines fusion specificity (McNew et al., 2000). However, the inevitable cycling of SNAREs between different compartments implies that this cannot be the only source of selectivity, and there is ample evidence that early docking events, prior to SNARE complex assembly, are also specific (Pfeffer, 1999). A second family of proteins, the Rab/Ypt monomeric GTPases, have been implicated in this process.
Rab/Ypts (11 members in the budding yeast Saccharomyces cerevisiae and >60 in humans) localize to different intracellular compartments and regulate transport between the various organelles by cycling between a GDP- and a GTP-bound state (Segev, 2001; Zerial and McBride, 2001). The most well-understood function of Rab/Ypts concerns their role in vesicle docking. GTP-loaded Rab/Ypts recruit effectors, soluble proteins or multiprotein complexes, that tether donor and acceptor membranes. Mammalian p115 is an effector of Rab1 in endoplasmic reticulum (ER) to Golgi transport, and its yeast homologue Uso1p is required for tethering ER vesicles to Golgi membranes in a Ypt1-dependent manner (Cao et al., 1998; Allan et al., 2000). Rab5 effectors include EEA1, a coiled-coil protein involved in docking of early endosomes (Christoforidis et al., 1999), and rabenosyn-5 (Nielsen et al., 2000), whose yeast homologue Vac1p is an effector of Ypt51p (also known as Vps21p) and is implicated in fusion with endosomes (Peterson et al., 1999; Tall et al., 1999). Recent studies have also provided direct links between Rab/Ypt effectors and the SNARE machinery: Vac1p binds the Sec1 homologue Vps45p, which in turn interacts with the syntaxins Pep12p and Tlg2p (Nichols et al., 1998; Abeliovich et al., 1999; Peterson et al., 1999; Tall et al., 1999), and similarly rabenosyn-5 binds the mammalian homologue of Vps45p (Nielsen et al., 2000). EEA1 binds to syntaxin 6 and 13 (McBride et al., 1999; Simonsen et al., 1999), and the HOPS complex, an effector of Ypt7 that contains the Sec1 homologue Vps33p, binds to the syntaxin Vam3p (Sato et al., 2000; Seals et al., 2000). These interactions suggest that, apart from their role in membrane tethering, Rab/Ypt effectors can have a direct role in the regulation of SNARE complex formation.
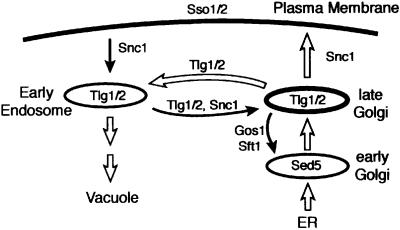
Transport to and from the Golgi requires the concerted function of SNAREs, Ypt GTPases and their accessory proteins, which include guanine nucleotide exchange factors and GTPase-activating proteins. In yeast, Ypt1p is required for docking of ER-derived vesicles with early Golgi compartments containing the SNARE Sed5p (Cao et al., 1998; Cao and Barlowe, 2000). The related pair Ypt31/32p has been suggested to act in exit from the Golgi (Benli et al., 1996; Jedd et al., 1997). Another GTPase associated with the Golgi is Ypt6p, the yeast homologue of mammalian Rab6. This protein is thought to be required for the delivery of vesicles to the late Golgi. In yeast, all known late Golgi membrane proteins cycle through an endocytic compartment and have to be retrieved constantly (Wilsbach and Payne, 1993; Bryant and Stevens, 1997; Lewis et al., 2000) (Figure 1). In ypt6 mutants, this process fails, and Golgi proteins such as Kex2p and the sorting receptor Vps10p are mislocalized to the vacuole; the vacuolar protease carboxypeptidase Y, which is sorted by Vps10p, is partially secreted as a result (Tsukada et al., 1999; Siniossoglou et al., 2000; Bensen et al., 2001).
Fig. 1. Recycling of late Golgi proteins in yeast. Overview of the main transport routes in the yeast Saccharomyces cerevisiae that are discussed herein. The locations and movements of relevant SNARE proteins are indicated.
The late Golgi SNAREs Tlg1p and Tlg2p also undergo recycling, and consequently are present in both Golgi and early endosomal membranes (Lewis et al., 2000). Thus vesicles carrying retrieved proteins back to the Golgi must distinguish late Golgi from endosomal membranes, even though vesicles, Golgi and endosomes all contain the same SNAREs. Ypt6p potentially could provide the necessary specificity, since we have shown that it is activated to the GTP form by a heterodimeric exchange factor (Ric1p–Rgp1p) that is a peripheral membrane protein restricted to the Golgi (Siniossoglou et al., 2000). How Ypt6p or Rab6 might regulate vesicle traffic previously was unknown. We show here that the GTP form of Ypt6p recruits a complex containing the Vps52, Vps53 and Vps54 proteins, previously shown to be required for retrieval of late Golgi proteins (Conibear and Stevens, 2000), and that this complex independently binds the N-terminal domain of the SNARE Tlg1p. This suggests a mechanism in which asymmetric localization of the exchange factor initiates a cascade of interactions that result in the selective docking and fusion of vesicles with the late Golgi.
Results
The VFT complex and Sgm1p bind to Ypt6:GTP
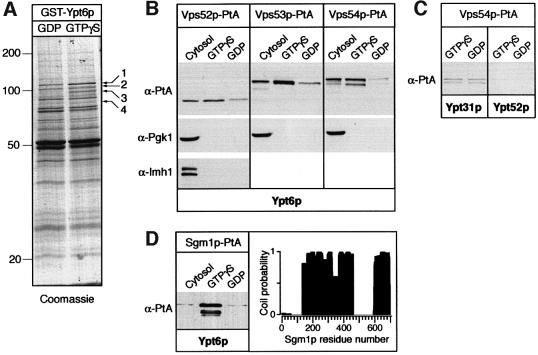
Endosome to Golgi recycling of membrane proteins is a poorly characterized transport pathway that depends, in yeast, on Ypt6p and its exchange factor complex Ric1p–Rgp1. To search for effectors of Ypt6p, we purified it as a recombinant GST fusion protein on beads, loaded it with GDP or GTPγS, and incubated the beads with yeast cytosol (see Materials and methods). Figure 2A shows that whilst many polypeptides bound to both forms of the protein, four appeared to be associated specifically with the GTP form. Mass spectrometry of peptides from band 1 showed that it contained Sgm1p, an 81 kDa protein of unknown function. Band 3 was identified as a mixture of two proteins: Mdr1p, a soluble GTPase-activating protein known to act on Ypt6p (Albert and Gallwitz, 1999), and Vps53p. Vps53p was shown recently to form a complex with two other proteins, Vps52p and Vps54p, and to be required for retrieval of proteins from endosomes to the Golgi (Conboy and Cyert, 2000; Conibear and Stevens, 2000). For brevity, we refer to this trimeric complex as the VFT (Vps fifty three) complex. Bands 2 and 4 have the sizes expected for the other VFT components.
Fig. 2. Ypt6p effectors. (A) Identification of proteins specifically bound to GST–Ypt6:GTPγS. Cytosol from a wild-type yeast strain was incubated with GST–Ypt6 affinity columns that had been pre-loaded with GDP or GTPγS and bound proteins eluted and analysed by SDS–PAGE followed by Coomassie staining as described in Materials and methods. Ypt6p effector proteins are numbered (1–4). Band 1 contains both Sgm1p and a second protein that binds in a nucleotide-independent manner. Band 3 corresponds to Vps53p and Mdr1p. (B) Western blot analysis of fractions eluted from GST–Ypt6:GTPγS or GST–Ypt6:GDP columns that had been incubated with cytosol from strains expressing Vps52p-PtA, Vps53p-PtA or Vps54p-PtA (strains SSY12, SSY13 and SSY14, respectively). Blots were probed with anti-PtA, anti-Pgk1p or anti-Imh1p antibodies. (C) As (B), but the columns contained GST–Ypt31p or GST–Ypt52p and were incubated with cytosol from a strain expressing Vps54p-PtA. The blot was probed with anti-PtA. (D) Left panel: binding of Sgm1p-PtA from cytosol to GST–Ypt6p as in (B). Right panel: the coiled-coil profile of Sgm1p as predicted using a 21 residue window and the program Coils (Lupas, 1996).
To confirm their binding to Ypt6p, we tagged Vps52p, Vps53p and Vps54p with protein A, and incubated cytosol from the appropriate strains with GST–Ypt6p beads. Figure 2B shows that each tagged protein bound preferentially to the GTP form of Ypt6p, whereas the cytosolic protein Pgk1p or the Golgi-associated peripheral membrane protein Imh1p (Munro and Nichols, 1999; Tsukada et al., 1999) did not bind. The VFT complex did not recognize specifically the GTP form of another Golgi GTPase, Ypt31p, or the endosomal GTPase Ypt52p (Figure 2C). To test whether the VFT–Ypt6p binding was direct, or required additional yeast proteins, we first affinity purified the VFT complex from yeast cells using a protein A tag that could be removed with tobacco etch virus (TEV) protease. Elution from IgG–Sepharose with TEV protease gave a VFT preparation contaminated only with TEV protease, some IgG and the coat protein from the yeast L-A virus, a common contaminant in these purifications (Figure 3A). Purified VFT, containing Vps54p-myc, bound to Ypt6p:GTP-containing beads (Figure 3B); silver staining of the gel confirmed that the contaminants did not (data not shown).
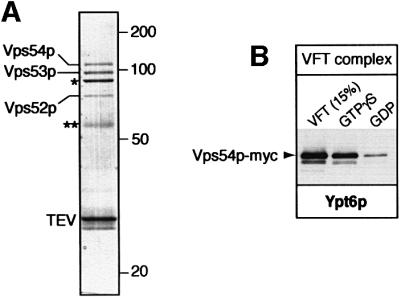
Fig. 3. The VFT complex binds directly to Ypt6p. (A) Purification of the VFT complex from yeast cells expressing Vps54p-PtA (strain SSY14). The complex was eluted from IgG–Sepharose with TEV protease, which cleaved off the PtA. Besides the protease (TEV), the contaminants are IgG (**) and the major coat protein of the yeast virus L-A (*). The identity of each band was confirmed by mass spectrometry. (B) The VFT complex, prepared from cells expressing Vps53-PtA and Vps54p-myc (strain SSY16) and purified by IgG–Sepharose, was incubated with GST–Ypt6p in the GTPγS or GDP form, eluted and detected with anti-myc antibodies as described in Materials and methods. The VFT lane is equivalent to 15% of the input material.
We also tagged Sgm1p with protein A and confirmed that it bound specifically to the GTP form of Ypt6p (Figure 2D). This protein contains two substantial regions of predicted coiled-coil (Figure 2D), and database searches show that proteins with similar overall structure and identifiable homology in the C terminal coiled-coil region exist in fission yeast (GenBank accesssion No. 7490693) and humans (GenBank accession No. 6005904), amongst others. The coiled-coil structure suggests a possible vesicle tethering function (Pfeffer, 1999; Segev, 2001). However, deletion of the SGM1 gene did not result in any noticeable trafficking defect. In particular, recycling of a green fluorescent protein (GFP)-tagged version of the exocytic SNARE Snc1p from the plasma membrane via early endosomes to the Golgi (Lewis et al., 2000) was not affected (data not shown), implying that the function of Sgm1p is dispensable for endosome–Golgi transport in vivo. Genetic removal of both Sgm1p and the Golgi coiled-coil protein Imh1p also produced no phenotype (data not shown). Presumably, other unknown proteins can substitute for the activity of Sgm1p. In contrast, mutation of the VFT complex impairs growth and interferes with protein retrieval from endosomes (Bensen et al., 2000; Conboy and Cyert, 2000; Conibear and Stevens, 2000). Its interaction with Ypt6p is thus likely to be of functional significance.
Direct binding of VFT to the N-terminal domain of Tlg1p
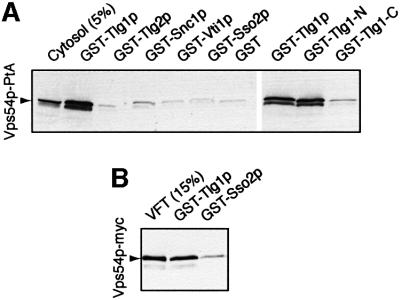
The phenotypes of ypt6 mutants are quite similar to those of mutants lacking the SNARE Tlg1p, which has been shown to be involved in the recycling of proteins from early endosomes to the Golgi apparatus, and to co-immunoprecipitate with the syntaxin Tlg2p and two other SNAREs, Snc1p and Vti1p (Holthuis et al., 1998a, b; Nichols et al., 1998; Lewis et al., 2000). To investigate possible interactions with the VFT complex, we expressed each of these four SNAREs, together with the plasma membrane syntaxin Sso2p and GST alone as controls, as GST fusion proteins in Escherichia coli, bound them to glutathione beads and incubated them with cytosol from cells expressing protein A-tagged Vps54p. As shown in Figure 4A, Tlg1p specifically bound the VFT complex whereas the other SNAREs did not. This interaction was direct, because VFT complex affinity purified from yeast also bound to Tlg1p-containing beads (Figure 4B). Since the VFT complex was purified via a protein A tag on Vps53p and detected using a myc tag on Vps54p, this result also confirms that it is Vps54p in the VFT complex, rather than free Vps54p, that binds to Tlg1p.
Fig. 4. Binding of the VFT complex to SNAREs. (A) Binding of Vps54p-PtA from cytosol to glutathione–Sepharose beads containing the indicated GST–SNARE fusions. The cytosol lane is equivalent to 5% of the material added to the binding reactions. The last three lanes are from a separate experiment; Tlg1-N contains amino acid residues 1–131 of Tlg1p; Tlg1-C contains residues 132–206. (B) As (A), but purified VFT complex, prepared as in Figure 3A, was used for the binding.
Though Tlg1p is now placed in a different class of SNAREs from the syntaxins (Bock et al., 2001), it has, like them, a bipartite structure: a C-terminal helix corresponding to the conserved SNARE–SNARE interaction domain and an N-terminal domain that is also predicted to contain helices. Expression of each of these domains separately as a GST fusion showed that the VFT complex bound to the isolated N-terminal domain as efficiently as to full-length Tlg1p, but did not bind to the C-terminal domain (Figure 4A). Thus, taken together, our in vitro binding experiments show that the VFT complex has specific binding sites both for Ypt6:GTP and for the N-terminal domain of Tlg1p.
Targeting of Ypt6p and its effectors is independent of the recycling of late Golgi membrane proteins
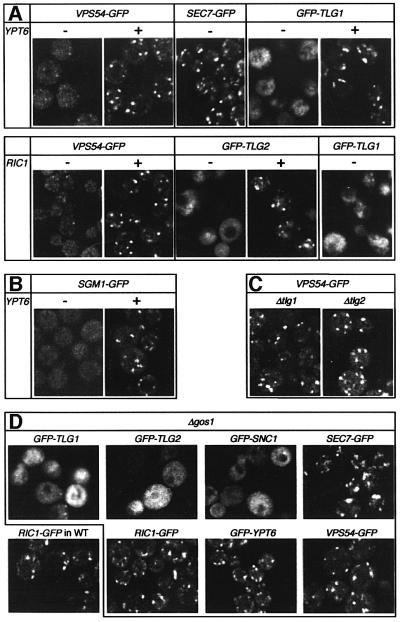
In vivo, the VFT complex has been reported to have a punctate distribution like that of the Golgi (Conboy and Cyert, 2000; Conibear and Stevens, 2000), and we confirmed this using functional GFP-tagged versions of Vps54p (Figure 5A) as well as Vps53p and Vps52p (data not shown). This distribution was changed to a much more dispersed pattern when Ypt6p was removed. Similar results were obtained when the Ypt6p exchange factor Ric1p was deleted, implying that the GTP form of Ypt6p is required for the correct localization of the VFT complex (Figure 5A). It should be stressed that mutant strains lacking Ypt6p can grow well and still contain punctate late Golgi structures, as shown by the location of a GFP-tagged form of the peripheral late Golgi marker Sec7p (Rossanese et al., 1999; Lewis et al., 2000) (Figure 5A). Sgm1p–GFP also showed a Ypt6p-dependent punctate localization, consistent with its in vitro binding properties (Figure 5B).
Fig. 5. Targeting of Ypt6p and its effectors does not depend on the recycling of late Golgi membrane proteins. (A–D) GFP-tagged proteins were expressed in various deletion mutants as indicated, and imaged by confocal microscopy. VPS54, SGM1, SEC7, RIC1 and YPT6 GFP fusions were expressed under the control of their respective promoters. TLG1, TLG2 and SNC1 fusions were under the control of the TPI1 promoter. Note that the punctate Golgi pattern of Vps54p depends on Ypt6p and Ric1p (A), but is unaffected by removal of Tlg1p, Tlg2p (C) or Gos1p (D). In contrast, the localization of Tlg1p and Tlg2p is affected by removal of Gos1p as well as Ypt6p or Ric1p (D). Under these conditions, Tlg1p and Tlg2p accumulate in a haze of vesicles and sometimes in more localized patches (see GFP–TLG1 in cells lacking RIC1), which correspond to the abnormally fragmented ‘vacuolar’ membranes that are found in ypt6 and ric1 mutants. Vacuolar delivery is a common fate for late Golgi proteins in these strains, but appears to be enhanced by the moderate overexpression of GFP–TLG1.
Since the VFT complex binds both Ypt6p and Tlg1p, the question arises as to whether its Golgi localization requires binding to one or to the other, or to both. The effects of ypt6 and ric1 mutations do not distinguish these possibilities, because removal of Ypt6p:GTP (the consequence of these mutations) also affects the location of Tlg1p (Figure 5A; see also Figure 1). Fusion of endosome-derived vesicles with the late Golgi is blocked, causing recycling integral membrane proteins to leave the Golgi and become trapped in endosome-derived vesicles and other membranes of the endosome/vacuole system (Tsukada et al., 1999; Siniossoglou et al., 2000; Bensen et al., 2001). However, genetic removal of Tlg1p, or Tlg2p, did not alter the VFT distribution (Figure 5C). Since these SNAREs are, like Ypt6p, required for traffic to the late Golgi, this eliminates the possibility that the effects of ypt6 and ric1 on VFT localization are due merely to the blocking of this process.
Though Ypt6p directs the VFT complex to the Golgi in the absence of Tlg1p, it could be that its normal function is simply to stabilize the Tlg1p–VFT interaction. To test their roles more definitively, we sought to separate Tlg1p from Ypt6p whilst retaining both in the cell. To do this, we blocked traffic to the late Golgi indirectly, using mutations in Sft1p and Gos1p. These SNAREs bind the early Golgi syntaxin, Sed5p, and are required for delivery of proteins from later in the secretory pathway to the early Golgi (Wooding and Pelham, 1998; McNew et al., 2000; Tsui et al., 2001) (Figure 1). As Golgi compartments mature, at least some late Golgi SNAREs must be delivered to the early Golgi, to initiate the delivery of other recycling late Golgi proteins. We found that in a deletion mutant of gos1 (Figure 5D), and also in a viable mutant allele of sft1 (data not shown), late Golgi membranes form that largely lack Tlg1p and Tlg2p and are thus unable to receive traffic from early endosomes. In these cells, GFP-tagged forms of Tlg1p and Tlg2p accumulate in a dispersed haze of transport vesicles, as does the exocytic SNARE Snc1p, which normally recycles to the Golgi via early endosomes. Thus the cells contain all the machinery for vesicle fusion with the late Golgi, but fusion cannot occur efficiently because the SNAREs are misplaced.
Despite the depletion of Tlg1p and other integral membrane proteins from the late Golgi in gos1Δ cells, the peripheral membrane proteins Sec7p, Ric1p, Ypt6p and Vps54p remain clearly punctate (Figure 5D). This suggests that the Ypt6p exchange factor, Ric1p, is localized at least in part by features of the Golgi other than its complement of integral membrane proteins. The exchange factor, in turn, can recruit Ypt6p, which recruits its effector, the VFT complex, to Golgi membranes.
In vivo interaction of VFT with Tlg1p in a YPT6-independent manner
We next sought evidence that the VFT complex does bind Tlg1p in vivo, by purifying the complex from cells, using Vps54p–protein A, and probing co-purifying proteins with antibodies. We were unable to detect significant interactions without cross-linking, but by treating spheroplasts with the cleavable cross-linker dithiobis-succinimidylpropionate (DSP), followed by detergent extraction of membranes, we could recover Tlg1p bound to Vps54p– protein A (Figure 6A). Cross-linking could also be observed with Tlg2p, though the fact that we did not detect direct VFT–Tlg2p interactions in vitro suggests that the interaction may be indirect, reflecting binding of Tlg2p to Tlg1p (Nichols et al., 1998). The amounts recovered were small, ∼1.5% of the SNAREs being cross-linked to the VFT complex by 3 mM DSP, but the binding was specific. There was no cross-linking to the early Golgi or plasma membrane syntaxins Sed5p or Sso1p, and neither Tlg1p nor Tlg2p bound to protein A alone (Figure 6A). We did not detect specific cross-linking of VFT to Snc1p or Vti1p, nor to Gos1p (data not shown). Similar results were obtained when Vps53p–protein A was used to purify the VFT complex (data not shown).
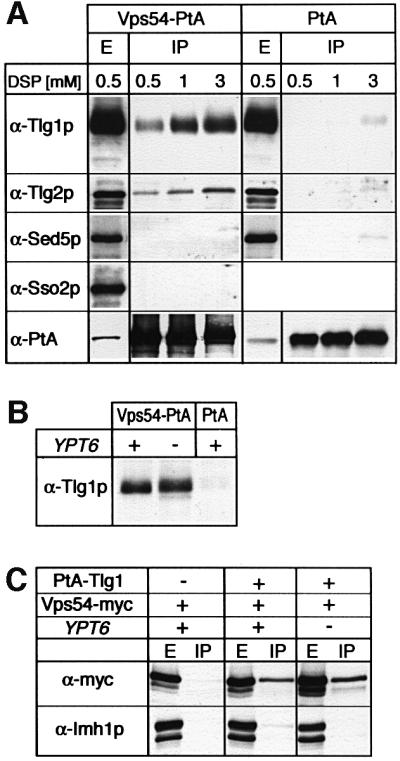
Fig. 6. The VFT complex can be cross-linked to late Golgi SNAREs in vivo. (A) Spheroplasts of cells expressing either Vps54-PtA (strain SSY14) or PtA (strain SSY17) were treated with 0.5, 1 or 3 mM of the cross-linker (DSP), detergent solubilized and the PtA fusion proteins affinity purified on IgG–Sepharose. Samples from the detergent-solubilized extracts (E, shown here for the 0.5 mM DSP extract) or the purified fractions (IP) were analysed by western blotting using anti-Tlg1p, Tlg2p, Sed5p, Sso2p and PtA antibodies. Note that the IP samples are overloaded relative to the E samples, as shown by the PtA blot. (B) Cells with (+) or without (–) YPT6, expressing Vps54p-PtA (strains SSY14 or SSY18, respectively) or PtA (SSY17), were spheroplasted, cross-linked with 1 mM DSP and analysed as in (A). (C) Cells expressing Vps54p-myc, PtA-Tlg1p (from centromere plasmids) and YPT6 as indicated (from left to right, strains SSY21, SSY20 and SSY19) were spheroplasted, cross-linked with 1 mM DSP, and PtA-Tlg1p was affinity purified on IgG–Sepharose as in (A). Samples from the extracts (E) and the affinity-purified fractions (IP) were analysed by western blotting using anti-myc and anti-Imh1p antibodies.
The VFT–Tlg1p cross-linking was unaffected by removal of Ypt6p (Figure 6B), indicating that it was not simply a consequence of recruitment of Vps54p to the Golgi, but represented an independent association of the VFT complex with the SNARE. In the absence of Ypt6p, Tlg1p is in vesicles and endosome-derived membranes (Figure 4A); the dispersed appearance of the GFP-labelled VFT complex under these conditions may thus in part reflect binding to vesicles.
To verify the VFT–Tlg1p interaction, we purified protein A-tagged Tlg1p from cells expressing myc-tagged Vps54p. After cross-linking, Vps54p could be detected clearly in the IgG–Sepharose eluate, but not in a control preparation from cells lacking protein A–Tlg1p (Figure 6C). As before, cross-linking of Tlg1p to Vps54p was at least as efficient in cells lacking Ypt6p. The peripheral late Golgi protein, Imh1p, was not cross-linked to Tlg1p, demonstrating the specificity of the interaction. These data suggest that the VFT complex could bridge acceptor and donor membranes by binding both to Ypt6p:GTP on Golgi membranes and to Tlg1p on vesicles (see Discussion).
Discussion
We have shown that a prime role for Ypt6p, once activated by GTP binding, is to recruit the VFT complex to late Golgi membranes. The VFT complex in turn binds to Tlg1p and allows vesicle fusion. Ypt6p also recruits the coiled-coil protein Sgm1p to Golgi membranes, and this may also contribute to vesicle tethering and docking. However, the absence of a phenotype in cells lacking Sgm1p means that its role remains to be established.
An important conclusion from this work is that although the presence of the appropriate SNAREs on Golgi membranes is essential for fusion, Ypt6p provides a second, equally important mechanism for identifying the target membrane (Figure 7). Specificity is determined ultimately by the localization of the Ypt6p exchange factor, Ric1p–Rgp1p. This is completely independent of the SNAREs, which can be removed or mislocalized without affecting the distribution of Ric1p. Other integral membrane proteins of the late Golgi are also unlikely to determine the presence of Ric1p, since the localization of such proteins itself depends on SNARE-mediated traffic (Holthuis et al., 1998a). What key feature of the Golgi is recognized by the exchange factor is unclear but, as with other organelles, it is possible that the lipid composition ultimately plays a role, either directly or indirectly—certainly this is important for Golgi function (Huijbregts et al., 2000).
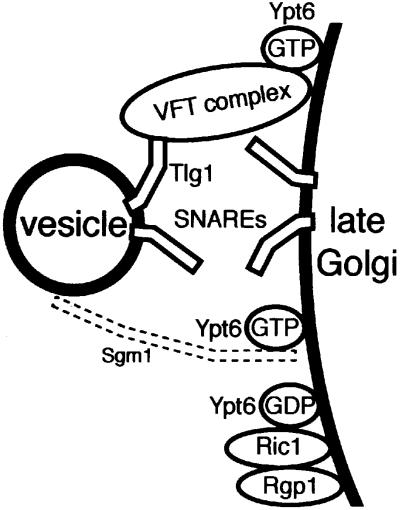
Fig. 7. Model for fusion of endosome-derived vesicles with the late Golgi. The Ric1p–Rgp1p complex activates Ypt6p by nucleotide exchange at the late Golgi membrane. Ypt6p:GTP recruits the VFT complex, which also interacts with the N-terminal domain of Tlg1p on vesicles (and/or on the Golgi membrane). SNARE engagement and fusion follow. Sgm1p also binds Ypt6p and may provide an additional tethering function, though this remains to be shown.
Ypt6p is linked to the SNAREs by the VFT complex, which behaves as a stable trimeric protein (Conibear and Stevens, 2000; our observations). The complex binds independently to Ypt6p and Tlg1p, although since individual subunits are not stable in either yeast or E.coli, we have not yet been able to determine whether the binding sites are on different polypeptides. We have shown that the complex is at least capable of binding to Tlg1p on vesicles, and thus could provide a direct way to select those vesicles that bear Tlg1p, which will include those derived from early endosomes but not, for example, primary endocytic vesicles. This potential targeting function of Tlg1p involves its N-terminal domain, and is distinct from the more conventional role of the SNAREs in selecting targets, namely the formation of C-terminal helical bundles with other SNAREs. SNARE–SNARE interactions are certainly crucial to the fusion event, but the SNARE–VFT–Ypt6p interaction can impose directionality on what would otherwise be, from the point of view of the SNAREs, a homotypic interaction.
Since Tlg1p normally is present on Golgi membranes as well as on vesicles, an alternative role for the VFT complex might be to bind to the SNARE on the Golgi and perhaps activate it. More detailed studies will be required to determine whether binding to Tlg1p in cis or in trans is preferred. However, in either case, stimulation of SNARE complex formation by VFT is a possibility. The structure of Tlg1p is not known, but like the syntaxins it has predicted amphipathic helices in its N-terminal domain. If these normally bind to the C-terminal helix that mediates SNARE–SNARE interactions, as in the syntaxin 1 structure (Misura et al., 2000), then binding of VFT to the N-terminus might have the effect of exposing the C-terminal interaction domain and hence promoting SNARE engagement.
If VFT recruitment is dependent upon Ypt6p, and this is the main function of Ypt6p, then VFT and ypt6 mutations should have similar phenotypes. Indeed, both result in temperature-sensitive growth, fragmented vacuoles and loss of Kex2p from the Golgi. However, while ypt6 mutants show only mild mis-sorting of carboxypeptidase Y (Tsukada et al., 1999), a stronger phenotype has been reported for vps52, vps53 and vps54 (Conibear and Stevens, 2000), though more modest effects were reported by others (Bensen et al., 2000; Conboy and Cyert, 2000). It is possible that the VFT complex has some residual affinity for the Golgi even in the absence of Ypt6p, and hence can to some extent act independently. Nevertheless, the general similarity of the phenotypes supports a close functional association of Ypt6p and the VFT complex.
The SNAREs Tlg1p, Tlg2p, Snc1p and probably Vti1p function not only in the Golgi but also in early endosomes, where they are thought to mediate the fusion of primary endocytic vesicles (Seron et al., 1998; Lewis et al., 2000). There is evidence to suggest that a separate set of accessory proteins may contribute to this endocytic function. Thus, EEA1, an effector of Rab5 that is present on early endosomes, binds to the mammalian Tlg1p homologue, syntaxin 6 (Simonsen et al., 1999). Furthermore Vac1p, an endosomal effector of the Rab5 homologue Ypt51p, binds to Vps45p, which in turn binds tightly to Tlg2p as well as being required for the function of the late endosomal syntaxin Pep12p (Nichols et al., 1998; Abeliovich et al., 1999; Peterson et al., 1999; Tall et al., 1999). Indeed, we have found recently that Vac1p, as well as Ypt51p or its relative Ypt52p, is required for the recycling of Snc1p through early endosomes; this is presumably due to their interactions with Tlg2p, since Pep12p is not required for this pathway (Lewis et al., 2000; M.Lewis and H.R.B.Pelham, unpublished observations). It thus seems that use of the same set of SNAREs at different locations is made possible by the actions of separate sets of Rab/Ypt proteins and their effectors. This may be an example of a general phenomenon: the Rab family has expanded far more than the SNARE family during evolution (Bock et al., 2001), suggesting that SNAREs often may participate in multiple fusion events controlled by distinct Rabs.
Human cells contain homologues not only of Tlg1p (syntaxin 6), Tlg2p (syntaxin 16) and Ypt6p (Rab6), but also of Ric1p, Sgm1p and all three members of the VFT complex, indicating conservation of much of the Golgi targeting machinery. However, it has been shown recently that subtly different isoforms of Rab6 exist, whose functions apparently differ (Echard et al., 2000). This complexity has made it difficult to understand the precise function of Rab6, which has been implicated both in traffic through the Golgi (Martinez et al., 1994) and in Golgi–ER transport (White et al., 1999). By analogy with yeast, we suggest that at least one of its functions may be to identify membranes of the Golgi or trans-Golgi network as a fusion target.
Materials and methods
Yeast strains and plasmids
The yeast strains used in this work are listed in Table I. Recombinant GST fusions were expressed using the pGEX6P2 plasmid (Pharmacia-Amersham). The following plasmids were used for yeast expression: YCplac111 and Ycplac33, ARS1/CEN4 vectors with the LEU2 and the URA3 marker, respectively (Gietz and Sugino, 1988); pUN100-(PNOP1)PtA, an ARS1/CEN4 plasmid expressing the PtA tag (Senger et al., 1998); pRS416-(PTPI)GFP–TLG2, a pRS416 plasmid expressing an N-terminal GFP–TLG2 under the control of the TPI1 promoter (kindly provided by M.Lewis), pRS315-(PTPI)GFP–TLG1, a pRS315 plasmid expressing an N-terminal GFP–TLG1 under the control of the TPI1 promoter (kindly provided by M.Black); YCplac111-RIC1–GFP, a YCplac111 plasmid expressing a C-terminal RIC1–GFP fusion (Siniossoglou et al., 2000); YCplac111-GFP–YPT6, a YCplac111 plasmid expressing an N-terminal GFP–YPT6 plasmid (Siniossoglou et al., 2000); and an integration construct to express SEC7–GFP from the chromosome (Rossanese et al., 1999).
Table I. Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα ura3 his3 leu2 trp1 suc2 lys2 | S.Emr |
| SSY12 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps52::HIS3 [YCplac111-LEU2-VPS52-PTA] | this study |
| SSY13 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps53::HIS3 [YCplac111-LEU2-VPS53-PTA] | this study |
| SSY14 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps54::HIS3 [YCplac111-LEU2-VPS54-PTA] | this study |
| SSY15 | MATα ura3 his3 leu2 trp1 suc2 lys2 sgm1::HIS3 [YCplac111-LEU2-SGM1-PTA] | this study |
| SSY16 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps54::HIS3 [YCplac111-LEU2-VPS53-PTA] [YCplac33-URA3-VPS54-MYC] | this study |
| SSY17 | MATα ura3 his3 leu2 trp1 suc2 lys2 [pRS315- LEU2-(PNOP1)-PTA] | this study |
| SSY18 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps54::HIS3 ypt6::TRP1 [YCplac111-LEU2-VPS54-PTA] | this study |
| SSY19 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps54::HIS3 ypt6::TRP1 [pUN100-LEU2-(PNOP1)-PTA-TLG1] [YCplac33-URA3-VPS54-MYC] | this study |
| SSY20 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps54::HIS3 [pUN100-LEU2-(PNOP1)-PTA-TLG1] [YCplac33-URA3-VPS54-MYC] | this study |
| SSY21 | MATα ura3 his3 leu2 trp1 suc2 lys2 vps54::HIS3 [YCplac33-URA3-VPS54-MYC] | this study |
Gene deletions and construction of fusion genes
The complete VPS52, VPS53, VPS54 and SGM1 open reading frames (ORFs) were deleted by generating two unique BamHI sites via PCR-mediated mutagenesis, one just after the start codon and the other just before the stop codon, removing the DNA between start and stop codon and inserting a BamHI fragment containing the HIS3 gene. The vps52::HIS3, vps53::HIS3, vps54::HIS3 and sgm1::HIS3 alleles, carrying 5′- and 3′-flanking sequences, were excised and transformed into the SEY6210 strain. To construct VPS52-, VPS53-, VPS54- and SGM1-PtA, GFP or myc C-terminal fusions, a BamHI site was introduced just before the stop codon of each gene by PCR and a BamHI fragment coding for the TEV protease cleavage site followed by two IgG-binding domains from Staphylococcus aureus protein A, GFP or myc was inserted. In all experiments, VPS52-, VPS53-, VPS54- and SGM1-PtA or GFP fusions were expressed from their own promoters on centromere vectors in the respective deleted strains. To construct the PtA-TLG1 fusion, the TLG1 ORF was cloned into the NdeI–BamHI sites of the pUN100-(PNOP1)PtA plasmid. The PtA-TLG1 fusion was expressed under the control of the NOP1 promoter from a centromeric plasmid with the LEU2 marker (pUN100). All fusions of the VPS genes and TLG1 were functional as judged by the complementation of the growth defects of the respective deleted strains.
Antibodies
The antibody used to detect protein A fusions was from Dako (catalogue No. Z0113). The anti-myc polyclonal antibody was from Santa Cruz (catalogue No. sc-789) and the anti-Pgk1p monoclonal from Molecular Probes (catalogue No. A-6457). Antibodies to Tlg1p, Tlg2p, Sed5p and Sso2p were as described earlier (Holthuis et al., 1998a). The antibody to Imh1p (Tsukada et al., 1999) was a gift from D.Gallwitz.
Mass spectrometic analysis
Protein identification by mass spectrometry of tryptic fragments was performed as previously described (Siniossoglou et al., 2000). Samples were analysed with a Voyager-DE-STR MALDI-TOF mass spectrometer (PerSeptive Biosystems). Database searches using peptide masses were performed with the Mascot program (http://www.matrixscience.com). Twenty-eight tryptic peptides were obtained from Sgm1p (sequence coverage 48%, mean mass error 41 p.p.m.), 14 peptides from Vps53p (sequence coverage 23%, mean error 17 p.p.m.) and 11 peptides from Mdr1p (sequence coverage 12%, mean error 4 p.p.m.).
Identification of effector proteins binding to Ypt6:GTP
Recombinant GST–Ypt6p was purified and loaded with GTPγS or GDP as described in Christoforidis and Zerial (2000) with modifications. The MC1061 E.coli strain, containing the pGEX6P2-YPT6 plasmid, was induced for 3 h with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were lysed and the supernatant was incubated with glutathione–Sepharose beads (Amersham Pharmacia) at 4°C. Beads were washed with phosphate-buffered saline (PBS) containing 1% Triton X-100, and with binding buffer [20 mM Tris–HCl pH 8.0, 110 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT)]. The beads were then washed with NE buffer (binding buffer containing 10 mM EDTA, 10 µM GTPγS or GDP) and incubated with NE buffer in the presence of 1 mM GTPγS or GDP for 30 min at room temperature. This was repeated twice more before the beads were washed once with NS buffer (binding buffer containing, 10 µM GTPγS or GDP) and then incubated with NS buffer in the presence of 1 mM GTPγS or GDP for 30 min at room temperature. For the preparation of the yeast cytosol, 7 g of spheroplasts from a wild-type strain (SEY6210) were lysed in 25 ml of binding buffer containing protease inhibitors. The extract was centrifuged at 100 000 g for 30 min at 4°C and the supernatant was dialysed against binding buffer. Binding reactions of GST–Ypt6:GTPγS or GST–Ypt6:GDP with yeast cytosol were performed for 2 h at 4°C in the presence of 100 µM GTPγS or GDP. Beads were then washed sequentially with 3 ml of binding buffer, 3 ml of binding buffer containing 220 mM KCl and 1 ml of 20 mM Tris–HCl pH 8.0, 220 mM KCl, 1 mM DTT. GST–Ypt6:GTPγS or GDP-interacting proteins were eluted with 300 µl of binding buffer containing 1.5 M KCl, 20 mM EDTA, 1 mM DTT and 5 mM GDP or 1 mM GTPγS, respectively, for 15 min at room temperature. Eluates were diluted with 20 mM Tris–HCl, concentrated by ultrafiltration and analysed by SDS–PAGE.
Affinity purification of PtA fusions and cross-linking
A vps54 deletion strain co-expressing Vps53p-PtA and Vps54p-myc was used to purify the VFT complex by IgG–Sepharose chromatography followed by TEV protease-dependent elution of the complex as previously described (Siniossoglou et al., 2000). In vivo cross-linking of the VFT complex to SNAREs was done as follows: 500 mg of spheroplasts from yeast cells expressing either Vps54p-PtA, PtA-Tlg1p or Vps54p-myc were resuspended in 5 ml of 25 mM potassium phosphate pH 7.4, 200 mM sorbitol containing protease inhibitors and 0.5, 1 or 3 mM DSP (Pierce), and incubated for 30 min at 4°C. The cross-linker was then quenched for 5 min with the addition of 150 mM Tris–HCl pH 7.4, cell suspensions were diluted with 10 ml of 150 mM KCl, 5 mM MgCl2, and Triton X-100 was added to a final concentration of 1%. Protein A fusions were purified by IgG–Sepharose chromatography and eluted either by low pH (Vps54p-PtA) or by TEV protease digestion (PtA-Tlg1p) as described in Siniossoglou et al. (2000). Cross-links were cleaved using 30 mM DTT in the SDS sample buffer.
In vitro binding experiments
The full-length cytosolic domains of Tlg1p (residues 1–206), Vti1p (1–193), Snc1p (1–93), Tlg2p (Holthuis et al., 1998a), Sso2p (1–273) and the N- or C-terminal domain of Tlg1p (residues 1–131 and 132–206, respectively) were expressed in E.coli as GST fusions using the pGEX6P2 vector, and recombinant proteins were purified on glutathione–Sepharose beads as described above. Spheroplasts from a strain expressing Vps54p-PtA were lysed in binding buffer, the extract was centrifuged at 100 000 g for 30 min at 4°C and 0.4 ml of the supernatant was incubated with the GST–SNARE beads for 2 h at 4°C. To test the binding of the purified VFT complex from yeast to either GST–Tlg1p and GST–Sso2p or GST–Ypt6:GTPγS and GST–Ypt6:GDP, an affinity-purified VFT complex prepared as described above was mixed with glutathione beads carrying the respective GST fusions in 1 ml of 20 mM Tris–HCl pH 8.0, 110 mM NaCl, 6 mM MgCl2, 0.1 mM EDTA, 1 mM DTT for 2 h at 4°C. In all cases, beads were washed four times with 0.5 ml of binding buffer and bound proteins were eluted with SDS sample buffer. An equivalent amount of GST fusion proteins was used in all binding experiments except for GST–Tlg2p, which was around four times lower in the experiment shown in Figure 4A. Repeated experiments did not detect significant binding of Vps54p-PtA to Tlg2p.
Acknowledgments
Acknowledgements
We thank Sew Peak-Chew and Farida Begum for expert protein analysis by mass spectrometry, Mike Lewis for reagents, strains and unpublished information, and Sean Munro, Michael Black, Fulvio Reggiori and Ewald Hettema for comments on the manuscript. S.S. was supported by a long-term postdoctoral fellowship from the Human Frontiers Science Program.
References
- Abeliovich H., Darsow,T. and Emr,S.D. (1999) Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE–Sec1p complex composed of Tlg2p and Vps45p. EMBO J., 18, 6005–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert S. and Gallwitz,D. (1999) Two new members of a family of Ypt/Rab GTPase activating proteins. Promiscuity of substrate recognition. J. Biol. Chem., 274, 33186–33189. [DOI] [PubMed] [Google Scholar]
- Allan B.B., Moyer,B.D. and Balch,W.E. (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science, 289, 444–448. [DOI] [PubMed] [Google Scholar]
- Benli M., Doring,F., Robinson,D.G., Yang,X. and Gallwitz,D. (1996) Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J., 15, 6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Bensen E.S., Costaguta,G. and Payne,G.S. (2000) Synthetic genetic interactions with temperature-sensitive clathrin in Saccharomyces cerevisiae: roles for synaptojanin-like Inp53p and dynamin-related Vps1p in clathrin-dependent protein sorting at the trans-Golgi network. Genetics, 154, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen E.S., Yeung,B.G. and Payne,G.S. (2001) Ric1p and the Ypt6p GTPase function in a common pathway required for localization of trans-Golgi network membrane proteins. Mol. Biol. Cell, 12, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J.B., Matern,H.T., Peden,A.A. and Scheller,R.H. (2001) A genomic perspective on membrane compartment organization. Nature, 409, 839–841. [DOI] [PubMed] [Google Scholar]
- Bryant N.J. and Stevens,T.H. (1997) Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J. Cell Biol., 136, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. and Barlowe,C. (2000) Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol., 149, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Ballew,N. and Barlowe,C. (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J., 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S. and Zerial,M. (2000) Purification and identification of novel Rab effectors using affinity chromatography. Methods, 20, 403–410. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., McBride,H.M., Burgoyne,R.D. and Zerial,M. (1999) The Rab5 effector EEA1 is a core component of endosome docking. Nature, 397, 621–625. [DOI] [PubMed] [Google Scholar]
- Conboy M.J. and Cyert,M.S. (2000) Luv1p/Rki1p/Tcs3p/Vps54p, a yeast protein that localizes to the late Golgi and early endosome, is required for normal vacuolar morphology. Mol. Biol. Cell, 11, 2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E. and Stevens,T.H. (2000) Vps52p, Vps53p and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol. Biol. Cell, 11, 305–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echard A., Opdam,F.J., de Leeuw,H.J., Jollivet,F., Savelkoul,P., Hendriks,W., Voorberg,J., Goud,B. and Fransen,J.A. (2000) Alternative splicing of the human Rab6A gene generates two close but functionally different isoforms. Mol. Biol. Cell, 11, 3819–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R.D. and Sugino,A. (1988) New yeast–Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene, 74, 527–534. [DOI] [PubMed] [Google Scholar]
- Holthuis J.C., Nichols,B.J., Dhruvakumar,S. and Pelham,H.R. (1998a) Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J., 17, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis J.C., Nichols,B.J. and Pelham,H.R. (1998b) The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol. Biol. Cell, 9, 3383–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts R.P., Topalof,L. and Bankaitis,V.A. (2000) Lipid metabolism and regulation of membrane trafficking. Traffic, 1, 195–202. [DOI] [PubMed] [Google Scholar]
- Jedd G., Mulholland,J. and Segev,N. (1997) Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol., 137, 563–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.J., Nichols,B.J., Prescianotto-Baschong,C., Riezman,H. and Pelham,H.R. (2000) Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell, 11, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. (1996) Prediction and analysis of coiled-coil structures. Methods Enzymol., 266, 513–525. [DOI] [PubMed] [Google Scholar]
- Martinez O., Schmidt,A., Salamero,J., Hoflack,B., Roa,M. and Goud,B. (1994) The small GTP-binding protein rab6 functions in intra-Golgi transport. J. Cell Biol., 127, 1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M., Rybin,V., Murphy,C., Giner,A., Teasdale,R. and Zerial,M. (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell, 98, 377–386. [DOI] [PubMed] [Google Scholar]
- McNew J.A., Parlati,F., Fukuda,R., Johnston,R.J., Paz,K., Paumet,F., Sollner,T.H. and Rothman,J.E. (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature, 407, 153–159. [DOI] [PubMed] [Google Scholar]
- Misura K.M., Scheller,R.H. and Weis,W.I. (2000) Three-dimensional structure of the neuronal-Sec1–syntaxin 1a complex. Nature, 404, 355–362. [DOI] [PubMed] [Google Scholar]
- Munro S. and Nichols,B.J. (1999) The GRIP domain—a novel Golgi-targeting domain found in several coiled-coil proteins. Curr. Biol., 9, 377–380. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Holthuis,J.C. and Pelham,H.R. (1998) The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur. J. Cell Biol., 77, 263–268. [DOI] [PubMed] [Google Scholar]
- Nielsen E., Christoforidis,S., Uttenweiler-Joseph,S., Miaczynska,M., Dewitte,F., Wilm,M., Hoflack,B. and Zerial,M. (2000) Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J. Cell Biol., 151, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M.R., Burd,C.G. and Emr,S.D. (1999) Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome. Curr. Biol., 9, 159–162. [DOI] [PubMed] [Google Scholar]
- Pfeffer S.R. (1999) Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol., 1, E17–E22. [DOI] [PubMed] [Google Scholar]
- Rossanese O.W., Soderholm,J., Bevis,B.J., Sears,I.B., O’Connor,J., Williamson,E.K. and Glick,B.S. (1999) Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J. Cell Biol., 145, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T.K., Rehling,P., Peterson,M.R. and Emr,S.D. (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell, 6, 661–671. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Eitzen,G., Margolis,N., Wickner,W.T. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N. (2001) Ypt and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol., 13, 500–511. [DOI] [PubMed] [Google Scholar]
- Senger B., Simos,G., Bischoff,F.R., Podtelejnikov,A., Mann,M. and Hurt,E. (1998) Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO J., 17, 2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seron K. et al. (1998) A yeast t-SNARE involved in endocytosis. Mol. Biol. Cell, 9, 2873–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Gaullier,J.M., D’Arrigo,A. and Stenmark,H. (1999) The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem., 274, 28857–28860. [DOI] [PubMed] [Google Scholar]
- Siniossoglou S., Peak-Chew,S.Y. and Pelham,H.R. (2000) Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J., 19, 4885–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall G.G., Hama,H., DeWald,D.B. and Horazdovsky,B.F. (1999) The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol. Biol. Cell, 10, 1873–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui M.M., Tai,W.C. and Banfield,D.K. (2001) Selective formation of Sed5p-containing SNARE complexes is mediated by combinatorial binding interactions. Mol. Biol. Cell, 12, 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M., Will,E. and Gallwitz,D. (1999) Structural and functional analysis of a novel coiled-coil protein involved in Ypt6 GTPase-regulated protein transport in yeast. Mol. Biol. Cell, 10, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. et al. (1999) Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol., 147, 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbach K. and Payne,G.S. (1993) Dynamic retention of TGN membrane proteins in Saccharomyces cerevisiae. Trends Cell Biol., 3, 426–432. [DOI] [PubMed] [Google Scholar]
- Wooding S. and Pelham,H.R. (1998) The dynamics of Golgi protein traffic visualized in living yeast cells. Mol. Biol. Cell, 9, 2667–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M. and McBride,H. (2001) Rab proteins as membrane organizers. Nature Rev. Mol. Cell. Biol., 2, 107–117. [DOI] [PubMed] [Google Scholar]