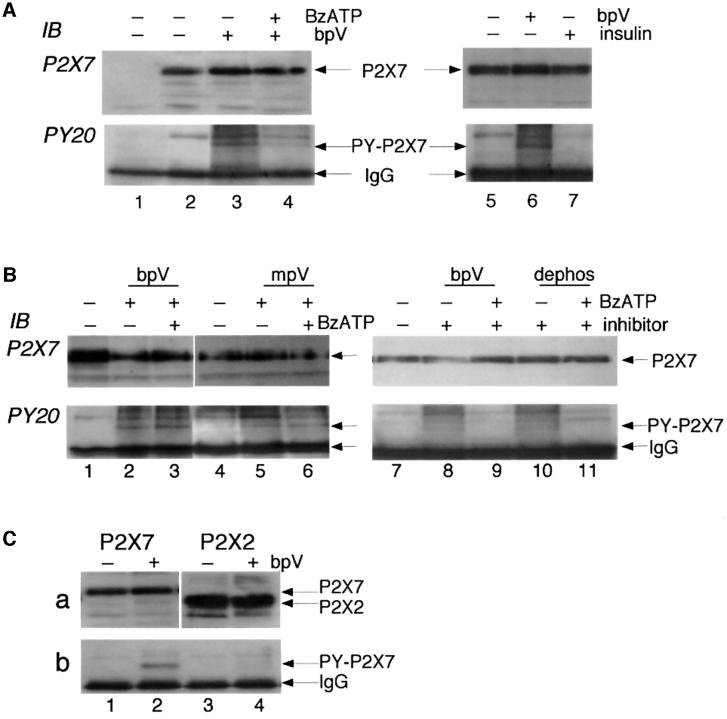
Fig. 2. Tyrosine phosphorylation of P2X7 receptor. (A) Membrane extracts from untransfected (lane 1, negative control) or P2X7 receptor-expressing HEK293 cells were immunoprecipitated with ecto-P2X7 Ab, and phosphotyrosine incorporation into the P2X7 protein was detected by probing with anti-phosphotyrosine Ab PY20. The blot was then stripped and re-probed with C-terminal anti-P2X7 Ab to confirm that the phosphotyrosine bands (lanes 3, 4 and 6) were the P2X7 receptor. No tyrosine phosphorylation was detected in control P2X7-expressing cells (lanes 2 and 5) but after 10 min incubation with the tyrosine phosphatase inhibitor bpV (100 µM) phosphotyrosine was clearly detected (lanes 3 and 6). The level was much reduced if the P2X7 receptor was activated for 10 min with BzATP (100 µM) prior to incubation with bpV (lane 4). (B) Similar experiments using phosphatase inhibitors mpV (100 µM, lanes 5 and 6) or 3,4-dephostatin (100 µM, lanes 10 and 11) also show tyrosine phosphorylation of P2X7 subunit in the presence of the phosphatase inhibitors (lanes 5 and 10), which is reduced when BzATP is added prior to application of phosphatase inhibitor (lane 11), but not when BzATP is added after the phosphatase inhibitor (lanes 3 and 6). (C) Tyrosine phosphorylation occurs on P2X7 receptor but not P2X2 receptor. Similar experiment to others performed on HEK cells transiently transfected with P2X7-EE (lanes 1 and 2) or P2X2-EE (lanes 3 and 4) receptors. Immunoprecipitation was with anti-EE Ab in the absence or presence of bpV as indicated. Anti-PY20 blotting detected phosphotyrosine-P2X7 after bpV treatment (b, lane 2) but no tyrosine phosphorylation of P2X2 receptor (b, lanes 3 and 4). Stripping and re-probing with C-terminal anti-P2X7 (a, lanes 1 and 2) or anti-P2X2 (a, lanes 3 and 4) confirms tyrosine phosphorylation of P2X7 but not P2X2 receptor even though there is greater expression of P2X2 protein.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.