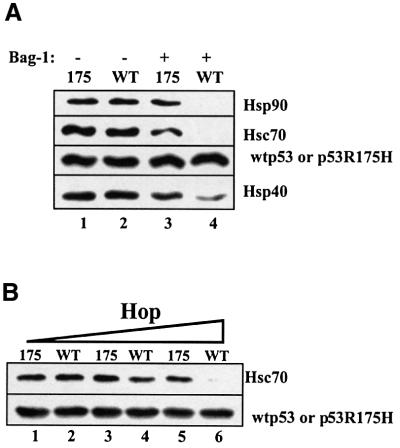
Fig. 8. Mutant p53, but not wild-type 53 forms a complex with multiple chaperones that displays resistance to Bag-1 dissociation. (A) Wild-type p53 (0.25 µM) or mutant p53R175H was incubated with 2 µM Hsc70, 1 µM Hsp40, 2 µM Hsp90, 0.4 µM Hop and 1 mM ATP (lanes 1–4) for 1 h at 25°C, followed by the addition of 4 µM Bag-1 (lanes 3 and 4) and a further incubation for 30 min at 25°C. p53–chaperone complexes were immunoprecipitated with monoclonal antibody Pab421 as described in Figure 1. (B) Wild-type p53 (0.25 µM) or mutant p53R175H was incubated with 2 µM Hsc70, 1 µM Hsp40, 2 µM Hsp90, 1 mM ATP and 0.1 µM Hop (lanes 1 and 2), 0.2 µM Hop (lanes 3 and 4) or 0.4 µM Hop (lanes 5 and 6) for 1 h at 25°C, followed by the addition of 4 µM Bag-1 (lanes 1–6) and a further incubation for 30 min at 25°C. p53–chaperone complexes were immunoprecipitated as above.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.