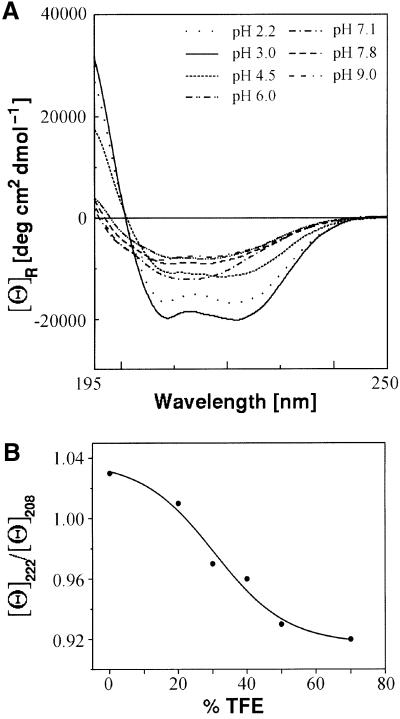
Fig. 3. Conformational state of the synthetic neck peptide Kn1. (A) The CD spectrum of the peptide Kn1 at varying pH values shows that an acidic environment below pH 4.5 induces an α-helical conformation, indicating the importance of protonated glutamic acid residues. (B) The ellipticity ratio [Θ]222/[Θ]208 of 1.03 in 50 mM phosphate buffer at pH 3, 20°C, indicates a coiled-coil formation, which is disrupted by increasing amounts of TFE, as seen by the sigmoidal decrease of [Θ]222/[Θ]208 to 0.96.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.