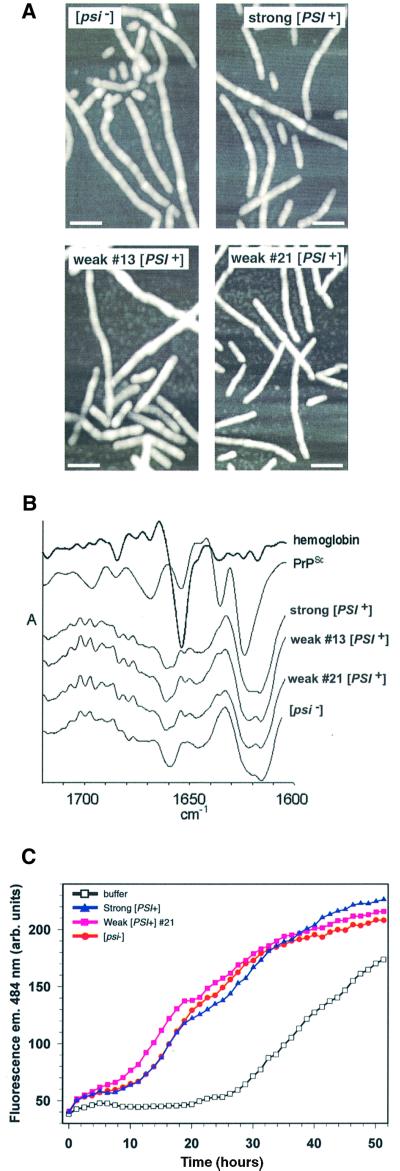
Fig. 4. Structural and biochemical characterization of fibers converted in the presence of Sup35PSI+ from different variants. (A) AFM images taken in tapping mode of fibers formed in the presence of Sup35PSI+ from strong or weak variants or with an identically prepared fraction from a [psi–] strain. The white bar represents 100 µm. (B) Second derivative attenuated total reflectance infrared spectra of NM fibers converted with strong [PSI+], weak [PSI+] (13 and 21) and [psi–] lysates. Second derivative spectra show negative deflections that correspond to positive absorbance maxima in primary spectra and provide a means of better resolving small differences between infrared spectra. For comparison, the spectra of hemoglobin (predominantlyα-helix) and PrPSc (mixed α-helix and β-sheet) are included. The amide I region is shown, which is sensitive primarily to the secondary structure of the polypeptide backbone. No consistent differences were observed between the four types of NM fiber in either the primary (not shown) or second derivative spectra. All of the NM fiber spectra showed predominant negative bands in the region dominated by β-sheet absorbances (∼1616–1640 cm–1); however, the pattern of these probable β-sheet bands was distinct from that of PrPSc. The other major NM fiber band was centered at ∼1660 cm–1, a wavenumber that is inconsistent with either β-sheet or α-helix but consistent with the presence of turn structure(s). (C) Determining whether the characteristic conversion efficiencies of Sup35PSI+of variants can be serially passaged to a second NM preparation. Sucrose P2 pellets from [psi–] and two [PSI+] variants (strong or weak 21) were used to prepare NM fibers that were subsequently diluted 1000-fold into another preparation of 10 µM soluble NM.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.