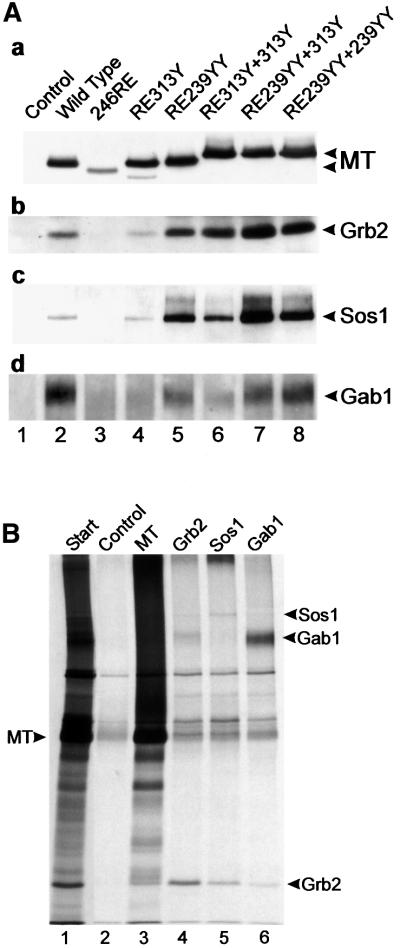
Fig. 6. (A) Cellular proteins associated with Grb2 bound to MT mutants. The MT mutants shown in Figure 3 were immunoprecipitated, separated and then blotted. Duplicate blots were then probed with a series of antibodies to detect MT-bound Grb2 and Grb2-bound Sos1 and Gab1. The antibody used is indicated to the right, together with the migration position of the particular polypeptide. The mutant used is indicated above each lane. A 10% acrylamide gel (a and b), or an 8% gel (c and d) was used to aid in polypeptide separation. Similar results were obtained in three separate experiments using the same cell lines. (B) Grb2, Sos1 and Gab1 were phosphorylated in vitro in MT immunoprecipitates. Lysates containing wild-type MT were immunoprecipitated with PAb762 and then incubated with [γ-33P]ATP (Amersham Pharmacia). The phosphorylated polypeptides were eluted with 1% SDS, renatured and immunoprecipitated with anti-MT, anti-Grb2, anti-Sos1 or anti-Gab1, and then separated by SDS–PAGE and autoradiographed. The migration of MT is indicated on the left and the associated proteins on the right. The antibody used is indicated above each lane. No antibody was added to the control lane and the small amount of polypeptides observed here represents either non-specific precipitation or a small amount of renaturation of the original precipitating antibody. The start lane is shown at a lower exposure to aid identification of the MT-bound polypeptides. The experiment was performed twice with similar results.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.