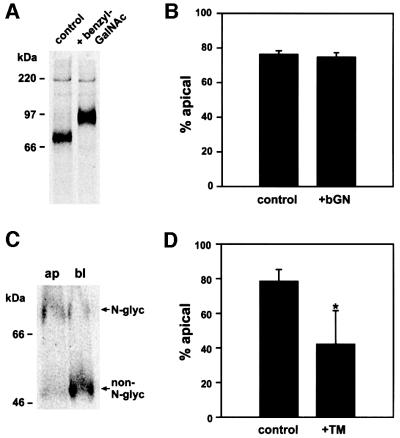
Fig. 9. Apical delivery of endolyn is independent of O-glycosylation, but dependent on N-glycosylation. (A) Endolyn-expressing MDCK cells were incubated with or without 4 mM benzyl-GalNAc (bGN) for 2 h and radiolabeled for 30 min (± drug). (B) Cells were drug-treated as above and steady-state binding of [125I]anti-endolyn was assessed as described in Figure 1C (mean ± range from two experiments). (C and D) Cells were radiolabeled for 3 h in the presence of 2 µg/ml tunicamycin (TM), chased for 1 h and then biotinylated at the apical or basolateral PM. In the example shown in (C), tunicamycin did not completely inhibit N-glycosylation, so that the reversed polarities of N-glycosylated and non-N-glycosylated endolyn are readily apparent (79.7% glycosylated and 30.6%, non-N-glycosylated endolyn at the apical PM). (D) Mean percentages of N-glycosylated (control) and non-N-glycosylated endolyn (tunicamycin-treated cells) at the apical surface (± SD of five experiments).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.