Abstract
Post-traumatic stress (PTS) encompasses a range of psychological responses following trauma, which may lead to more severe outcomes such as post-traumatic stress disorder (PTSD). Identifying early neuroimaging biomarkers that link brain function to PTS outcomes is critical for understanding PTSD risk. This longitudinal study examines the association between brain dynamic functional network connectivity (dFNC) and current/future PTS symptom severity, and the impact of sex on this relationship. By analyzing 275 participants’ dFNC data obtained ~2 weeks after trauma exposure, we noted that brain dynamics of an inter-network brain state link negatively with current (r=−0.197, pcorrected= 0.0079) and future (r=−0.176, pcorrected= 0.0176) PTS symptom severity. Also, dynamics of an intra-network brain state correlated with future symptom intensity (r = 0.205, pcorrected = 0.0079). We additionally observed that the association between the network dynamics of the inter-network and intra-network brain state with symptom severity is more pronounced in female group. Our findings highlight a potential link between brain network dynamics in the aftermath of trauma with current and future PTSD outcomes, with a stronger effect in female group, underscoring the importance of sex differences.
Introduction
Post-traumatic stress disorder (PTSD) may develop in individuals who have experienced or witnessed a traumatic event, such as military warfare, sexual or physical assault, accidents, or natural disasters 1. Symptoms of PTSD include distressing thoughts, flashbacks, avoidance of reminders, changes in mood and cognition, and increased arousal, which can significantly impact an individuals’ life 2. Biological markers or biomarkers may be able to identify those who are more likely to develop PTSD following a traumatic incident 3,4. Early identification of such individuals might allow for prompt treatment and preventive measures, potentially minimizing the severity and duration of PTSD symptoms. Furthermore, these markers may help in the development of tailored treatment methods, the optimization of therapeutic treatments, and the long-term monitoring of therapy response 5.
In recent years, there has been a significant increase in the exploration and advancement of neuroimaging-based markers for identifying vulnerability to PTSD 6,7. This emerging field shows great potential in the rapid development of tools for early identification and intervention 8. Studies utilizing neuroimaging techniques have uncovered notable alterations in brain function among individuals with PTSD. These alterations are marked by atypical functional network connectivity (FNC) patterns, as observed in resting-state functional magnetic resonance imaging (fMRI) studies 9–11. Specifically, these patterns are seen in various brain regions, including the hippocampus 12, amygdala 13, visual network14, and prefrontal cortex13 in individuals with PTSD. This underscores the extensive influence of trauma on brain networks. Furthermore, several studies have successfully utilized resting-state fMRI functional connectivity to predict the severity of PTSD symptoms 15–18. Additionally, two recent studies revealed the ability to predict future symptom severity in participants with PTSD by analyzing resting-state fMRI data obtained after the trauma had occurred 19,20.
It has been assumed that brain FNC remains quasi-static or invariant over long periods of time, leading many previous studies to focus solely on static FNC (sFNC) while ignoring the brain dynamics during rest. However, challenging this assumption, a relatively new concept called dynamic FNC (dFNC) has emerged 21–25. A dynamic approach recognizes that FNC during the relatively short length of resting-state fMRI scans can exhibit temporal variations, thereby highlighting the importance of studying the dynamic aspects of FNC 26. Unlike sFNC, dFNC offers greater sensitivity in capturing the spontaneous adaptations that occur in response to various cognitive and mental conditions 27. By considering the spontaneously fluctuating nature of neural signals across different temporal scales, dFNC allows for a more sophisticated evaluation of brain activity 28.
Considering the dynamic nature of FNC in resting-state fMRI, several studies have explored dFNC in the context of PTSD in recent years 29–32. However, none of these studies have examined the capability of dFNC to link with the future PTSD symptom severity. In addition, previous research indicates that women are two to three times more likely than men to develop PTSD 33. Despite this, there has been a notable absence of studies that examine the potential effects of sex on the relationship between dFNC variables and the severity of current or future PTSD symptoms.
In the present study, we aim to build upon previous research on dFNC in the context of PTSD. Specifically, we investigated the link between dFNC variables and future PTSD symptom severity. Additionally, we explored the potential effects of sex on the association between dFNC variables and both current and future symptom severity. As past studies have demonstrated, biological sex is not the primary determinant of the various neurophenotypes associated with adverse post-traumatic outcomes. Instead, a range of other factors such as low socioeconomic status or SES, including income 34,35, housing quality 36 , and broader socioeconomic conditions, area deprivation index or ADI 37 also significantly influence the risk and severity of PTSD. To address the contribution of these factors, we also included them as covariates in our analysis. Finally, our study aimed to identify specific brain states that underlie risk and protective mechanisms related to PTSD.
To accomplish these goals, we utilized the dataset from the Advancing Understanding of Recovery after Trauma (AURORA) project 38. In the AURORA study, understanding whether dFNC variables derived from resting-state fMRI early after a trauma can link with future PTSD symptom severity is crucial. This is especially true since neuroimaging was conducted approximately two weeks after the traumatic event, at a time when acute stress disorder may be assessed, but before the diagnosis of PTSD can be made. This timing allows us to investigate the potential of dFNC variables as early biomarkers for PTSD and evaluate their predictive capability for the severity of PTSD symptoms at a later stage.
Results
Participants
Data for the current analyses were collected as part of the multisite emergency department (ED) AURORA study. The AURORA study represents a significant research effort aimed at enhancing our understanding, prevention, and recovery strategies for individuals who have undergone a traumatic event. In AURORA study, trauma-exposed civilians brought to one of 29 participating EDs across the United States were recruited for this large, longitudinal study (details in38). This study involved around 3,000 participants from the AURORA project, who provided clinical data at various intervals: 2 weeks (WK2), 4 weeks (WK4), 3 months (M3), 6 months (M6), and 12 months (M12) as illustrated in Fig. 1A. Additionally, neuroimaging data from ~400 participants were collected at WK2 from five different scanning locations, which include Atlanta (Georgia), Belmont (Massachusetts), Philadelphia (Pennsylvania), St. Louis (Missouri), and Detroit (Michigan). The recruitment for this study took place between September 2017 and June 2021 (Final freeze 4 Psychometric release at 22/09/2021). We excluded those with low-quality resting-state fMRI and missing clinical information at the imaging acquisition date. This process resulted in 275 participants (181 female participants) being included in this analysis. Table 1 presents the demographic characteristics of the participants included in this study. Additionally, Supplementary Fig.1 illustrates the distribution of PCL-5 scores at various time points for the participants.
Fig. 1: Data collection procedure and analytic pipeline:
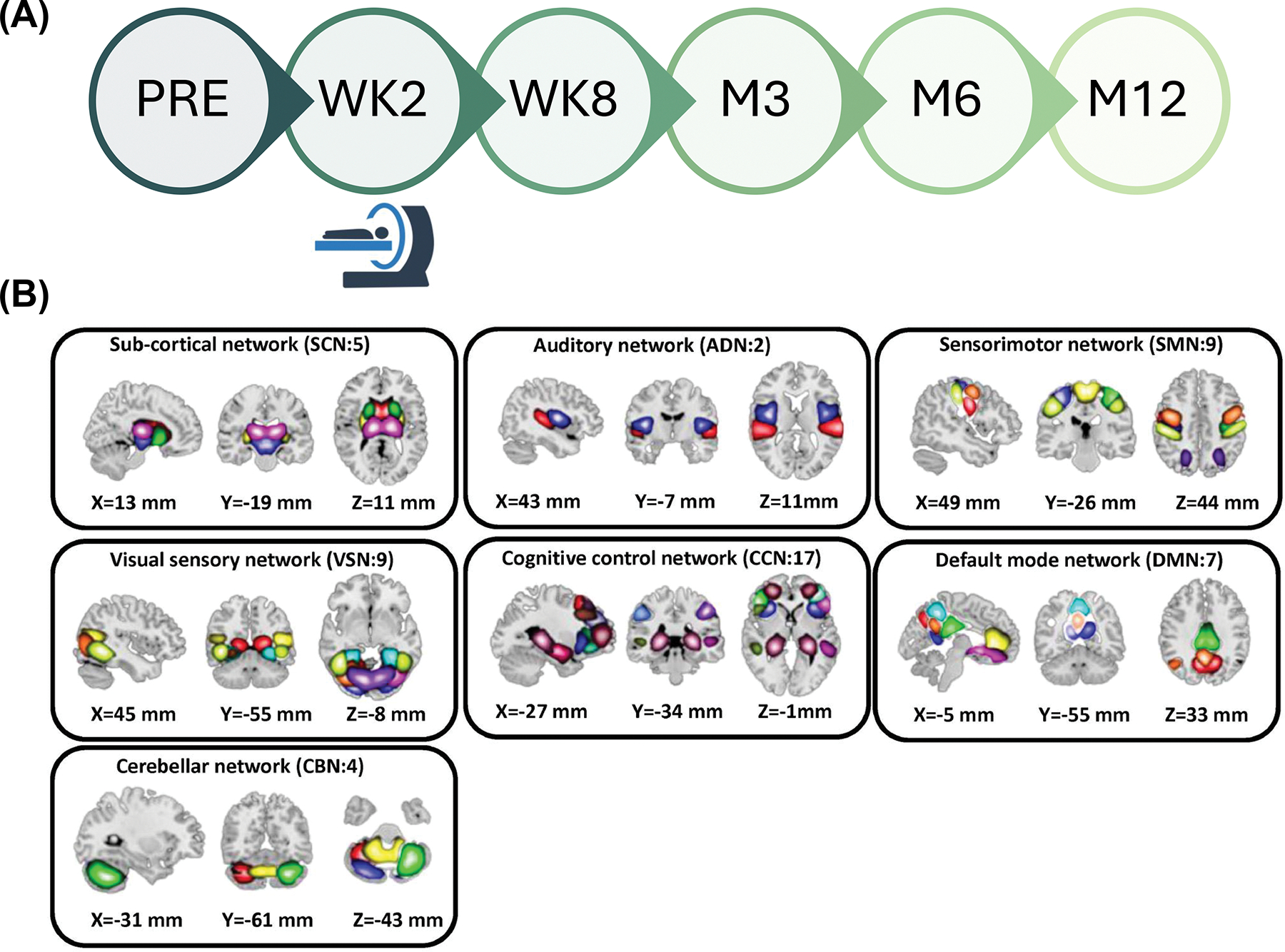

A) The PTSD Checklist for DSM-5 (PCL-5) was utilized to evaluate PTSD symptoms at various time points, encompassing pre-trauma (PRE), week 2 (WK2), week 8 (WK8), month 3 (M3), month 6 (M6), and month 12 (M12). During the study visit at WK2 a subgroup of participants underwent MRI scans, either in the morning or the afternoon. B) We utilized the NeuroMark pipeline to extract robust intrinsic connectivity networks (ICNs), totaling 53 components, which demonstrate consistent replication across independent datasets. These 53 distinct components were initially identified through group-ICA analysis using the NeuroMark template. These components were subsequently categorized into seven distinct networks, which include the subcortical network (SCN), auditory network (ADN), visual sensory network (VSN), sensorimotor network (SMN), cognitive control network (CCN), default mode network (DMN), and cerebellar network (CBN). C) Dynamic functional network connectivity (dFNC) analytic pipeline: Step 1: Initially, the time-course signal of 53 intrinsic connectivity networks (ICNs) was identified through group-ICA in the Neuromak template. Subsequently, the identified 53 ICNs were subjected to a taper sliding window segmentation to calculate FNC. Each subject yielded 210 FNCs, each with a size of 53 × 53. Step 2: To cluster the FNCs into three distinct groups, the FNC matrices were vectorized and concatenated, followed by the utilization of k-means clustering with correlation as the distance metric. Step 3: From the state vector, occupancy rate (OCR) was computed for each subject, resulting in a total of three OCR variables for each subject. In order to investigate the relationship between OCRs with the PTSD clinical measure (i.e, PCL-5), we used GLM to compute the associations, taking into account factors such as age, sex, years of education, scanning site, income, marital status, employment status, type of truama, and percentile ADI. The resulting t-statistics from this analysis were then converted to correlation (r) values.
Table 1.
Participant demographics and clinical information
| Characteristics | Mean (SD) or N (%) |
|---|---|
|
| |
| Demographic characteristics | |
|
| |
| Age | 34.55(12.78) |
| Sex assigned at birth, male/female | 94 (34.18%)/181(65.82%) |
| Race/ethnicity* | |
| Hispanic | 42 (15.27%) |
| White | 85 (30.91%) |
| Black | 131 (47.64%) |
| Others | 15 (5.45%) |
| Missing | 2 (0.73%) |
| Years of education | 15.16(2.31) |
| Income level | |
| <$19,000 | 74 (26.91%) |
| $19,001-$35,000 | 85 (30.91%) |
| $35,001-$50,000 | 40 (14.55%) |
| $50,001-$75,000 | 30 (10.91%) |
| $75,001-$100,000 | 17 (6.18%) |
| >-$100,000 | 20 (7.27%) |
| Missing | 9 (3.27%) |
| Trauma Type | |
| Motor Vehicle Collision | 197 (71.64%) |
| Physical Assault | 29 (10.55%) |
| Fall < 10 feet or from unknown height | 14 (5.09%) |
| Non-motorized Collision | 11 (4%) |
| Animal-related | 7 (2.55%) |
| Fall ≥10 Feet | 4 (1.45%) |
| Sexual Assault | 2 (0.73%) |
| Burns | 1 (0.36%) |
| Incident Causing Traumatic Stress Exposure to Many People |
1 (0.36%) |
| Poisoning ** | 0 (0%) |
| Other | 9 (3.27%) |
|
| |
| Clinical characteristics | |
|
| |
| PCL-5 score | |
| WK2 (N=275) | 30.12 (17.58) |
| WK8 (N=243) | 26.60 (17.30) |
| M3 (N=226) | 23.53 (17.40) |
| M6 (N=208) | 21.00 (17.33) |
| M12 (N=176) | 20.33 (17.93) |
Self-reported . SD: standard deviation, WK2: 2 weeks after trauma, WK8: 8 weeks after trauma, M3: 3 months after trauma, M6: 6 months after trauma, M12: 12 months after trauma.
None of the participants involved in this study experienced poisoning trauma, although poisoning has been reported as a type of trauma in the broader AURORA group, accounting for around 2% of cases.
Three distinct dFNC states were identified
After calculating the dFNC of each participant, we grouped their dFNC into three different dynamic network connectivity states (Fig.1B). Fig. 2 presents an overview of the identified states. Each state represents 1378 connectivity measures among seven networks across the entire brain. These networks included subcortical network (SCN), auditory network (ADN), sensorimotor network (SMN), visual network (VSN), cognitive control network (CCN), default-mode network (DMN), and cerebellar network (CBN). The top panel highlights three distinct dFNC states, while the bottom panel shows the data with connectivities between −0.3 and 0.3 removed for clarity. State 2 and state 3 exhibit a stronger positive connectivity among sensory networks, including visual, auditory, and sensorimotor networks. Conversely, in state 1, we observed more disconnections among these networks. We observed an increase in within-CCN connectivity and enhanced connectivity between the DMN and sensory networks in state 3. Additionally, we noted a greater connectivity between the CBN and SCN in state 3 compared to the other two states. Overall, our analysis suggests that state 2 and state 3 exhibit characteristics of inter-network states, evidenced by the increased connectivity across the seven networks. In contrast, state 1 is indicative of an intra-network state, as it demonstrates predominantly within-network connectivity patterns. Supplementary Fig. 2 provides further insights into the differences in FNC between states.
Fig. 2: Three dynamic functional connectivity states identified in AURORA dataset.
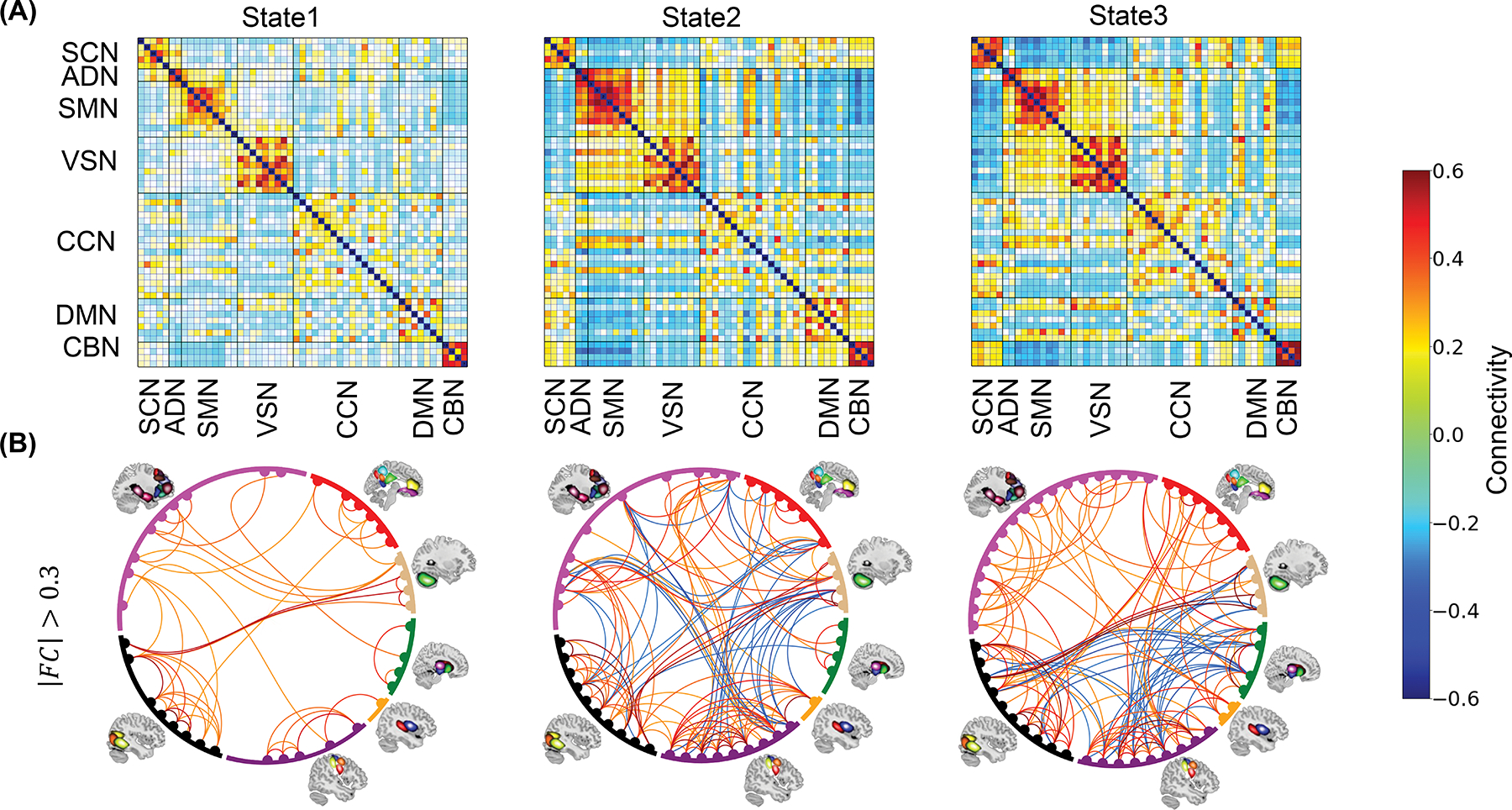
A) Three dynamic functional connectivity (dFNC) state identified using k-means clustering method for N=275 incldung both PTS and non-PTS individual. B) To enhance clarity, the dFNC state displayed by removing connectivities with values between −0.3 and 0.3. States 2 and 3 exhibit stronger positive connectivity among sensory networks (visual, auditory, and sensorimotor). State 1, on the other hand, shows more disconnections within these networks. State 3 demonstrates increased within-CCN connectivity and enhanced connectivity between the DMN and sensory networks compared to state 1 and satte 2. State 3 also exhibits greater connectivity between the CBN and SCN compared to the other two states. Overall, our analysis identifies states 2 and 3 as inter-network brain states while state 1 appears to be an intra-network brain state based on connectivity patterns. The color bar indicates the strength of the connectivity. SCN: Subcortical network; ADN: auditory network; SMN: sensorimotor network; VSN: visual network; CCN: cognitive control network; DMN: default-mode network; and CBN: cerebellar network.
Dynamic FNC occupancy rates link with PCL-5 scores
By utilizing the three identified brain states for the entire group and the state vector, estimated for each individual, which represents the state of the brain network at any given time point, we calculated three occupancy rates (OCRs) for each participant. The OCR of each state represents the proportion of time each participant spends in that state (see Method Section and Supplementary Fig. 3). Fig. 3A shows the correlation between OCRs and PCL-5 scores at various time points. The associations were computed using General Linear Model (GLM) accounting for age, sex, years of education, scanning site, income, marital status, employment status, type of trauma, and percentile ADI, and the resulting t-statistics were transformed to correlation (r). A positive significant association was found between the OCR of state 1 and the PCL-5 scores at M3 (r = 0.205, β= 0.0042, SE=0.0012, 95% CI: 0.0018~0.0066, pcorrected = 0.0079, N=226 after excluding sample with missing scores, see Table 1). These results indicate that the participants with higher PTSD symptom severity spend more time in state 1, which is indicative of an intra-network brain state.
Fig. 3: Dynamic functional network connectivity occupancy rates (OCRs) link with PCL-5 .

We employed a General Linear Model to explore the association between OCRs and PCL-5 scores using data from all participants at WK2 (N=275), WK8 (N=243), M3 (N=226), M6 (N=208), and M12 (N=176). We included age, sex at birth, years of education, income, employment status, marital status, scanning site, type of trauma, and percentile ADI as covariates. In the sex-stratified analysis, sex was excluded as a covariate. With three predictors and five time points, we have 15 tests. These partial correlations were analyzed using a two-sided test to assess the significance of associations in both directions. In each panel, all 15 p-values have been adjusted for multiple comparisons using FDR correction. A) We found a positive association between the OCR of state 1 and PCL-5 scores at M3 (r =0.205, β = 0.0042, SE=0.0012, 95% CI: 0.0018–0.0066, pcorrected=0.0079, N=226). We also found a significant negative association between the OCR of state 3 and PCL-5 scores of WK2 (r=−0.197, β=−0.0033, SE=0.0009, 95% CI: −0.0052~−0.0013, pcorrected =0.0079, N = 275), and between state 3 OCR and PCL-5 scores at M3 (r=−0.176, β=−0.0032, SE= 0.0011, 95% CI: −0.0054~ −0.0011, pcorrected=0.0176, N=226). B) A positive association is observed between the OCR of state 1 and PCL-5 scores both WK2 (r=0.187, β= 0.0034, SE=0.0014, 95% CI: 0.0001~0.0045, pcorrected=0.044, N=181) and M3 (r=0.224, β=0.0044, SE=0.0014, 95% CI: 0.00180–0.0066, pcorrected=0.019, N=154). Conversely, a negative correlation is seen between the OCR of state 3 and PCL-5 scores at both WK2 (r=−0.269, β=−0.0043, SE=0.0011, 95% CI: −0.0052~−0.0013, pcorrected = 0.004, N=181) and M3 (r=−0.208, β= −0.0036, SE=0.0013, 95% CI: −0.0055~−0.0011, pcorrected = 0.014, N=154). C) We did not find any significant result for the male group. Time points: WK2 (week 2 after trauma), WK8 (week 8 after trauma), M3 (month 3 after trauma), M6 (month 6 after trauma), and M12 (month 12 after trauma). The color bar represents correlation strength, with solid boxes indicating significant results after FDR correction and dashed boxes marking significant results that did not survive correction.
We observed significant negative association between the OCR of state 3 and the PCL-5 scores at WK2 (r=−0.197, β= −0.0033, SE=0.0009, 95% CI: −0.0052~- 0.0013, pcorrected= 0.0079, N=275). We also found a negative correlation between state 3 OCR and PCL-5 of M3 (r=−0.176, β=−0.0032, SE=0.0011, 95% CI: −0.0054~ −0.0011, pcorrected = 0.0176, N=226). This indicates that individuals with higher PCL-5 scores spent less time in state 3, which is indicative of an inter-network brain state. Overall, our findings highlight the relationships between the OCR and PCL-5 scores, suggesting potential connections between dFNC variables and symptoms of PTSD at different time points after trauma.
Sex modulates OCRs and PCL-5 scores relationship
To examine the influence of sex on the relationship between OCRs and PCL-5 scores, we conducted GLM analyses for male (N=94) and female (N=181) participants, separately. In these analyses, we included age, years of education, scanning site, income, marital status, employment status, type of trauma, and percentile ADI as covariates. The correlation results between OCRs and PCL-5 scores for female and male group are presented in Fig. 3B and Fig. 3C, respectively. While no significant association was found between OCRs and PCL-5 scores in the male group, we did observe significant associations between the OCR of state 1 and state 3 with PCL-5 scores at WK2 and M3 in female group. We observed a positive association between the OCR of state 1 and PCL-5 scores at WK2 (r=0.187, β= 0.0034, SE=0.0014, 95% CI: 0.0001~0.0045, pcorrected = 0.044, N=181) and M3 (r=0.224, β=0.0044, SE=0.0014, 95% CI: 0.0018~0.0066, pcorrected = 0.019, N=154). We also identified a negative correlation between the OCR of state 3 and PCL-5 scores at week 2 (r=−0.269, β= −0.0043, SE=0.0011, 95% CI: −0.0052~−0.0013, pcorrected = 0.004, N=181) and month 3 (r=−0.208, β= −0.0036, SE=0.0013, 95% CI: −0.0055~−0.0011, pcorrected = 0.014, N=154). Additionally, OCR of state 3 showed a negative link with M12 PCL-5 (r=−0.154, β= −0.0031, SE=0.0015, 95% CI: −0.0054~0.0003, puncorrected = 0.039, N=117) . However, this link was not significant after FDR correction. To confirm that the stronger correlation observed in female group is not merely due to their larger sample size compared to male group, we compared the correlations between OCR of state 1 and state 3 with PCL-5 scores at WK2 and M3 for both groups. We employed Fisher’s z-transformation to compare the correlations, calculating the standard errors to ensure precision. Our results indicated a significant difference between female and male groups for the correlation with state 1 OCR (|Z-test statistic| = 2.1262, p = 0.033), and similarly for state 3 OCR (|Z-test statistic| = 2.1029, p = 0.035). This suggests that the relationships between OCR of state 1 and state 3 with PCL-5 scores at WK2 are significantly different between the sexes.
PTS and non-PTS group generate similar dFNC states
We categorized participants into posttraumatic stress or PTS (N=124) and non-PTS (N=151) groups based on their WK 2 PCL-5 scores, with a cutoff point of 31. Those scoring above 31 were classified as PTS, while those below were considered non-PTS 39. We used the term PTS instead of PTSD because the classification was based on PCL-5 scores at the time of imaging (i.e., WK2), before an official PTSD diagnosis till WK8. We then examined state pattern differences between the two groups by performing separate k-means clustering analyses on their dFNC data. Fig. 4 demonstrates a notable similarity in brain states between the PTS and non-PTS groups, as anticipated. We quantified the similarity by calculating the Pearson correlation coefficient between corresponding states’ FNC. The correlations between state 1 of the non-PTS group and state 1 of the PTS group, state 2 of the non-PTS group and state 2 of the PTS group, and state 3 of the non-PTS group and state 3 of the PTS group were 0.9632 (N = 1378, where N is number of connections, p~0), 0.9880 (N = 1378, p~0), and 0.8938 (N = 1378, p~0), respectively (see Fig. 4A and 4B). The p-value, displayed as zero in MATLAB, indicates a very small value, suggesting strong statistical significance and reinforcing the robustness of our findings. Comparing the OCR of states in the non-PTS and PTS groups, we found a consistent pattern: state 1 consistently showed the highest OCR, while state 2 exhibited the lowest OCR in both groups (Fig. 4C and 4D). These results suggest a consistent OCR pattern across states in both groups, indicating a high degree of similarity in identified brain states between the non-PTS and PTS groups. Additionally, our findings that individuals with PTS tend to spend more time in state 1 compared to those without PTS corroborate our main finding that have established a connection between the heightened OCR of this state and PCL-5, hinting at the potential clinical relevance of this brain state in PTS.
Fig. 4: Both non-PTS and PTS group generate similar dynamic functional connectivity (dFNC) state.

A) the dFNC states identified only in non-PTS group (N=151). B) the dFNC states identified only in PTS group (N=124). C) The OCR in different state of non-PTS group ( N=151). D) The OCR in different state of PTS group ( N=124). The bar plot show the mean of OCR and the error bar shows the ±SD (standard deviation) from the mean. The color bar indicates the strength of the connectivity. SCN: Subcortical network; ADN: auditory network; SMN: sensorimotor network; VSN: visual network; CCN: cognitive control network; DMN: default-mode network; and CBN: cerebellar network.
Discussion
Our current research aimed to investigate the significance of temporal changes in brain connectivity, measured by dynamic functional network connectivity (dFNC), in indicating the presence and severity of PTSD symptoms. Additionally, we examined the influence of sex-specific differences on the predictive ability of these connectivity measures. Our results indicate that the amount of time spent in an inter-network brain state serves as a protective factor against PTSD, whereas time spent in an intra-network brain state is linked to a higher PTSD symptom severity. Furthermore, we observed that the association between the duration spent in the indentifed states and PCL-5 is more pronounced in the female group.
Dynamic FNC offers an enhanced predictive power compared to static FNC (sFNC), supplying an additional layer of information about the severity of symptoms in brain disorders over time, a level of detail not attainable by its static counterparts 40–42. For instance, a recent study demonstrated that a classification model relying on dFNC variables surpassed the performance of other classification models in patients diagnosed with multiple sclerosis 42. In another study involving participants with PTSD, the temporal variability, as captured by dFNC, demonstrated a higher classification accuracy than the model obtained only by sFNC variables 41. Our findings extend beyond previous dFNC research in PTSD 29–32 by demonstrating that brain network states can not only correlate with current symptom severity but also link with future PTSD symptoms. This ability to predict future symptom severity is particularly beneficial as it may enable earlier intervention strategies, tailored treatment plans, and potentially prevent the progression of the disorder 43,44. Moreover, the significant sex-specific differences in connectivity patterns we observed have not been detailed to this extent in earlier dFNC studies, providing new insights into how sex may influence the link between dFNC variables and the pathophysiology of PTSD.
In our study sample, comprising participants exposed to traumatic events, we analyzed dFNC and differentiated three distinct brain network states. Two out of the three states (i.e., state 2&3) exhibited a higher degree of integration in the sensory network, while state 1 demonstrated a more disconnected sensory network. State 3 manifested the strongest connectivity within the CCN, within the CBN, and between the CBN and the SCN. Moreover, we found that state 1 was characterized by intra-network connectivity, while the other two states exhibited inter-network connections with both strong negative and positive connectivity among brain networks. These observations collectively highlight that brain networks display substantial dynamism, a characteristic they maintain even without the presence of external stimuli as has been observed in other brain disorders 21–25,29,40. Additionally, we investigated whether the dynamics of brain networks in participants with PTS differed from those in the non-PTS group. Upon separately analyzing data from both groups of participants, we observed that each group generated similar dFNC states, as expected and observed in other disorders45. This suggests that the dynamic nature of brain networks persists irrespective of PTS, highlighting the potential complexities and resilience of the brain’s network dynamics in the face of trauma and related disorders.
A prior study, employing the same population as the current research, demonstrated that the static functional connectivity between the left dorsolateral prefrontal cortex (DLPFC) and the arousal network (AN), as well as between the right inferior temporal gyrus (ITG) and the default mode network (DMN), could predict both WK2 and M3 PCL-5 scores 20. In the current study, we found that the whole-brain OCRs estimated from dFNC link with the PCL-5 at the time of neuroimaging data collection (referred to as WK2), as well as the PCL-5 scores 10 weeks post-data collection (referred to as month 3 or M3). Our new analyses contribute to a deeper understanding of the neurobiological mechanisms underlying PTSD by looking at brain network dynamics.
Specifically, we found that participants with higher PCL-5 scores tend to spend more time in an intra-network brain state, referred to as state 1. Importantly, the amount of time spent in this state was found to link with future symptom severity at M3 (Fig. 3A). Supplementary Fig. 4 provides additional insights into the relationship between the OCR of state 1 and PTSD symptom severity. State 1 is characterized by reduced connectivity among sensory networks, including visual, auditory, and sensory motor networks. Furthermore, our results confirmed that spending more time in an inter-network brain state (state 3) is negatively correlated with PCL-5 scores at WK2 and M3 (Fig. 3A). State 3 is characterized by increased connectivity among sensory networks, suggesting enhanced information exchange and integration between these networks. Previous studies have consistently reported alterations in visual processing, as well as auditory processing, in individuals with PTSD 46,47. Multiple neuroimaging studies have demonstrated alterations in the functioning of the visual, auditory, and motor cortex among participants with PTSD 47–49. Notably, abnormal activation in the visual cortex during picture viewing tasks has been observed in these individuals 47. Furthermore, significant alterations in visual processing have been identified within the ventral visual stream, which is responsible for processing object properties 47. This suggests that PTSD may affect the specific components of the visual system involved in object recognition and perception, as previous findings, including those from the AURORA study, highlight a role for structural integrity of the ventral visual stream in the development of PTSD 50,51. Our current findings, in conjunction with previous reports of subtle deficits in sensory networks, particularly the visual sensory system in PTSD, provide compelling evidence that disruptions in information integration among sensory networks are closely linked to the severity of PTSD symptoms50–53. Enhancing the connectivity and integration within these networks could potentially serve as a therapeutic target for mitigating symptom severity and improving outcomes in individuals with PTSD 54.
In addition to the sensory networks, our findings reveal that state 1 is characterized by relatively lower within-CBN connectivity and between CBN and SCN connectivity (i.e., CBN/SCN) compared to the other two states. This observation aligns with previous structural neuroimaging studies that have reported reduced cerebellar volumes in individuals with PTSD 55,56. Furthermore, functional neuroimaging studies have provided corresponding evidence by demonstrating alterations in neural activity and functional connectivity of the cerebellum in PTSD 57. Our new finding, that participants with higher PCL-5 scores spent more time in the state characterized by lower CBN connectivity, adds another layer of information to the understanding of temporal network patterns associated with CBN in PTSD. This suggests that alterations in cerebellar connectivity patterns may play a role in modulating symptom severity and could serve as potential markers for the disorder.
In the subsequent analysis, we investigated the influence of sex on the relationship between brain network dynamics and symptom severity. We observed that the association between OCRs, and PCL-5 scores was more prominent in female group. Specifically, the correlation between state 1 OCR and WK2 PCL-5 as well as the correlation between state 3 OCR and WK2 PCL-5 was statistically significant within the female group, and the strength of this correlation was notably higher among female compared to male group (Fig. 3B and C). It is worth noting that previous studies have extensively explored the role of sex in the development of PTSD, with emerging evidence suggesting differences in symptomatology and underlying neurobiology between male and female groups 33,58. In line with these findings, our results further support the notion that the identified dFNC biomarkers, particularly when correlating with symptom severity, are stronger in female participants; this could potentially reflect the higher prevalence of PTS/PTSD in this demographic.
Recent large-scale genomic studies show that women of European and African ancestry may have higher heritability for PTSD than men, suggesting that genetic factors may also play a significant role in the disorder’s development, particularly in interaction with sex differences 59,60. However, it’s important to note that biological sex is not the primary determinant of the various neurophenotypes associated with adverse post-traumatic outcomes; other factors such as low socioeconomic status also play a significant role 34,35. To avoid a narrow focus on sex alone, our analysis took into consideration all available socioeconomic and demographic factors from the dataset. This approach allowed us to conduct a comprehensive analysis of the connection between OCRs and PTSD symptom severity, specifically considering the sex effect. Additionally, women’s risk for PTSD is partially determined by the fact that they experience sexual traumas more frequently. For example, a study shows that women exhibit almost twice the PTSD symptoms in sexual assault survivors61. However, in the AURORA dataset, the type of trauma does not play a major role in driving sex differences. The traumas are primarily motor vehicle collisions (MVCs) for both women and men, yet sex differences in dFNC link with PTSD sympthom severity are still observed.
Our findings highlight the potential of inter-network connectivity as a protective mechanism against PTSD. Specifically, our results suggest that transitioning the brain from a risk state (state 1) to a protective state (state 3) could be therapeutically beneficial. This insight is particularly applicable to closed-loop therapies such as closed-loop neuromodulation 62 and neurofeedback63,64, which can be tailored to induce such state transitions. State-dependent brain stimulation, which adjusts its parameters based on the current brain state, offers a promising approach to dynamically target and ameliorate high-risk states effectively 65–67. Administering neuromodulation when the brain is in a high-risk state and transitioning it to a more protective state could enhance therapeutic outcomes by leveraging the brain’s natural functional network dynamics 68. While the implementation of real-time, state-specific interventions presents technical challenges , including the real-time analysis of brain states and concurrent neuromodulation 69, the potential to mitigate PTSD symptoms preemptively could transform early intervention strategies.
Several limitations should be acknowledged while interpreting the present findings. The overall sample size was relatively modest, and the sample sizes amongst the comparison groups (male group vs. female group) were not the same. Furthermore, participants who completed all scans and had more complete datasets may differ from those who did not complete all scans, making it unclear if the results apply to dropouts who may be at higher risk for PTSD after trauma. In this study, we examined dFNC in individuals with PTS and a non-PTS group, both of whom were exposed to trauma. To gain a comprehensive understanding, further research is required to directly compare the dFNC variables among the PTSD group, a group of healthy individuals exposed to a traumatic event, and a group of healthy individuals who have not undergone any traumatic experiences. However, we assume that healthy individuals exposed to trauma could serve as a more suitable control group for those with PTSD, facilitating our understanding of the underlying neural processes of PTSD. Due to the data-driven approach employed in our study, which utilizes group-independent component analysis (ICA) 70 analysis to identify independent components, we do not have direct measurements of amygdala dynamics. However, we have identified one of the 53 components as a proxy for amygdala activity. Supplementary Fig. 5 provides more details about the dynamics of the amygdala in our study population, as analyzed in our pipeline. Additionally, in this study, we investigated the relationship between dFNC variables and the severity of PTSD symptoms at various time points. However, to enhance our understanding, future research should compare dFNC variables among groups exhibiting different PTSD trajectories during a one-year assessment. In our study, we utilized the initial neuroimaging data available from the AURORA study, which was collected two weeks post-trauma, before any PTSD diagnosis at week 8. Given that the AURORA study also gathered neuroimaging data at six months post-trauma, future research would benefit from examining the dFNC patterns using the resting-state fMRI data from this later time point. Such analysis could yield more profound insights into the evolving brain dynamics associated with PTSD. Finally, we observed low correlation values between dFNC variables and PTSD symptom severity, ranging from −0.269 to +0.224. This modest correlation may be influenced by several factors: the limited informativeness of the dFNC variables compared to other brain variables such as structural—a finding similarly reported in the previous AURORA study, albeit with different brain variables 58,71. Additionally, the relatively small sample size in our study might contribute to the low correlation values. However, previous PTSD studies with larger sample sizes have also reported low correlations between brain variables and symptom severity 72. Minimal variance in brain variables among trauma-exposed individuals may further contribute to these results.
Conclusions
In summary, our investigation into the dFNC of civilians recently exposed to trauma revealed distinct patterns in brain network dynamics. Our findings indicate that the duration participants spent in certain brain network states can link with both their current and subsequent PCL-5 scores. Specifically, we identified that spending time in an intra-network brain state is associated with higher PCL-5 scores, while engagement in an inter-network brain state correlates with lower PCL-5 scores. Furthermore, our analysis highlighted the role of multiple brain networks encompassing the visual, auditory, sensory-motor, and cerebellar networks, in PTSD. We also observed a stronger association between brain dynamics and PCL-5 scores in female group compared to the male group. By incorporating sex-specific disparities, tailoring interventions and treatment strategies accordingly, we can potentially develop more effective and personalized approaches for PTSD.
Methods
Inclusion and ethics statement
The study was conducted in accordance with ethical guidelines and received approval from the Institutional Review Board (IRB) at the University of North Carolina (IRB no. 1707–03) on 12 May 2017, covering multiple sites. Additional sites either entered into reliance agreements or conducted parallel IRB reviews. Participants provided written informed consent prior to participation. An independent medical monitor evaluated and approved the procedures for handling any cases of clinical deterioration reported by participants or identified by study staff. This monitor also reviewed detailed reports of participant interactions prepared by experienced clinicians.
Study population
Participants were recruited as part of the multi-site Emergency Department (ED) AURORA study 38 . The study targeted individuals who had experienced a traumatic event necessitating an ED evaluation, with recruitment occurring within 72 hours of the event 38. This cohort of early post-trauma participants was selected to explore pivotal changes in neurobiology and brain function that could heighten the risk for trauma-related psychopathology in the subsequent weeks or months. The study aimed to enroll a demographically representative sample of the US population without restrictions on demographic variables such as sex, gender, race, or ethnicity.
In this study, the participants who experienced incidents like a car accident, a high fall (>10 feet), a physical assault, sexual violence, or mass casualty incident were considered to have experienced trauma. The inclusion criteria include: 1) aged between 18 and 75 years old, 2) being alert and oriented at the Emergency Department (ED), 3) having the ability to speak and write English fluently, 4) having no cognitive impairment, 5) having the ability to use the smartphone for > 1-year post-enrollment. Exclusion criteria included solid organ damage, severe bleeding, a requirement for a chest tube, and the likelihood of being admitted for longer than 72 hours. The study eventually included 2,943 AURORA participants with clinical item-level data, recruited between September 2017 and Jne 2021, marking the final data freeze for psychometric release (Freeze 4.0 dataset at 22/09/2021). Participants recruited at one of the ED locations for the AURORA study, which directed participants to one of five ‘deep phenotyping’ sites, were invited to undergo MRI scans. These scans were conducted either in the morning or afternoon, approximately two weeks following the traumatic event (i.e., WK2). After thorough preprocessing and quality checks, data from 275 participants were included in our study (see Supplementry Fig. 6).
Race and Ethnicity
The Race/Ethnicity parameter reflects the participant’s background based on self-reported data collected during the initial ED baseline survey. This variable is an integer ranging from 1 to 4, categorizing ethnic backgrounds as 1 for Hispanic, 2 for Non-Hispanic White, 3 for Non-Hispanic Black, and 4 for Non-Hispanic Other. Classification is determined from responses to two survey questions—one about Hispanic, Latino, or Spanish origin and another concerning race. An algorithm uses these self-reported responses to assign participants to one of these groups, or marks the data as missing if responses are incomplete.
Clinical measures
The PTSD Checklist for DSM-5 (PCL-5) was administered to assess PTSD symptoms at multiple time points, including week 2 (WK2), week 8 (WK8), month 3 (M3), month 6 (M6), and month 12 (M12), as depicted in Fig. 1A. It is important to emphasize that different time frames were considered for each of the time points: the 2-week (WK2) assessment reflected symptoms experienced over the past two weeks, while assessments from week 8 (WK8) onwards considered symptoms over the past 30 days. This longitudinal assessment allows for a comprehensive understanding of the participants’ PTSD symptomatology throughout the study duration. Table 1 summarizes the demographic and clinical characteristics of the participants included in this study. Additionally, to distinguish individuals with posttraumatic stress (PTS) from those without PTS in WK2 of the study, we employ a threshold for the PCL-5 at 31. Participants with a PCL-5 score greater than 31 are classified as having PTS, while those with a score less than 31 are considered non-PTS 39. It is important to note that we refer to this group as having PTS and not PTSD, as the PTSD diagnosis was made in W8, while we used the WK2 PCL-5 scores to identify these two groups. Supplementary Fig.1 illustrates the distribution of PCL-5 scores for individuals included in this study at various time points. Also, a detailed comparison of clinical and psychological metrics across sex in the Non-PTS and PTS groups is presented in Supplementary Table 1. Additionally, Supplementary Fig.7 displays the number of PTSD and non-PTSD participants diagnosed with PTSD during subsequent assessments at WK8, and M3, M6, and M12 after traumatic event.
Types of traumatic events in the AURORA study
The AURORA study meticulously classifies traumatic events into specific categories to facilitate detailed analysis and understanding. Motor Vehicle Collisions may involve participants inside, on top of, or struck by various motorized vehicles like motorcycles and ATVs. Non-Motorized Collisions include incidents involving bicycles and skateboards. Physical Assault encompasses intentional injuries inflicted by another person, while Sexual Assault covers any sexual contact, ranging from groping to rape. Falls are categorized by height, with distinctions made between falls from above and below ten feet, noting that a fall from ten feet is typically equivalent to falling from a one-story building. Incidents causing traumatic stress exposure to many people refer to large-scale disasters that affect the participant along with others, such as plane crashes or natural disasters. Poisoning includes the ingestion or inhalation of toxic substances; Burns cover injuries from thermal, electrical, chemical sources, radiation, or friction; and Animal-related traumas involve reactions to stings or bites. This structured classification aids in the tailored analysis and support for those affected by various types of traumatic experiences.
Imaging acquisition protocol
Participants underwent a thorough screening process before undergoing scanning, which involved checking for any contraindications to magnetic resonance imaging (MRI) or other exclusion criteria. For female participants and those who could potentially be pregnant, a pregnancy test was administered prior to entering the MRI environment. MRI scans were conducted using 3T Siemens scanners at each site. While the scan sequences remained largely consistent across imaging sites, some variations in sequence parameters were present due to differences in hardware. The imaging protocol for each site is outlined in Supplementary Table 2. The resting-state imaging procedure lasted approximately 9 minutes, during which participants were instructed to keep their eyes open. They were asked to focus on the white cross displayed at the center of the screen and maintain a state of stillness throughout the imaging session 20.
Preprocessing
We corrected the differences in image acquisition times between slices using the statistical parametric mapping (SPM12 @ https://www.fil.ion.ucl.ac.uk/spm/) default slice timing routines. The slice acquired in the middle of the sequence was chosen as the reference slice. The subject’s head movement was then corrected using a rigid body, and 3-dimensional brain translations and 3-dimensional rotations were estimated. Next, the imaging data were resampled to 3 × 3 × 3 mm3. and spatially normalized to the Montreal Neurological Institute (MNI) space using the echo-planar imaging (EPI) template and the SPM toolbox’s default bounding box. The fMRI images were then smoothed using a Gaussian kernel with a full width at half maximum (FWHM) of 6 mm. It should be emphasized that while participants in this study have also been featured in other AURORA analyses and resting-state studies20,73, the current analyses are distinct. Additionally, the preprocessing approach diverges from the standard protocols commonly employed in AURORA research in order to align with methodologies used in our other work. A similar preprocessing approach has been employed in several of our previous studies 23–25,45,74.
Extracting independent components using Neuromark
We applied a hybrid Neuromark framework to extract the meaningful networks for each subject. The Neuromark framework is based on the Neuromark template derived from two large datasets including the human connectome project (HCP: https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release, 823 subjects after the subject selection) and genomics superstruct project (GSP: https://dataverse.harvard.edu/dataverse/GSP, 1005 subjects after the subject selection). This framework has been successfully implemented to many studies with a wide range of brain imaging markers identified across different brain diseases 23–25,45,74. Details of the construction of the templates can be found in our previous Neuromark paper 75.
The Neuromark template consists 53 independent components (ICs), which were grouped into seven functional networks based on anatomic and functional prior knowledge. These networks included subcortical network (SCN), auditory network (ADN), sensorimotor network (SMN), visual network (VSN), cognitive control network (CCN), default-mode network (DMN), and cerebellar network (CBN) (Fig.1B) 76. All 53 ICs and their coordination are shown in Supplementary Table 3. We used these priors (i.e., the Neuromark_fMRI_1.0 template, available in GIFT @ http://trendscenter.org/software/gift and on the TReNDS website @ http://trendscenter.org/data) to run a fully automated ICA analysis in GIFT v4.0.5.14 70. We further: 1) detrended linear, quadratic, and cubic trends, 2) conducted multiple regression on the six realignment parameters and their temporal derivatives, 3) despiked detected outliers, and 4) applied a low-pass filter (cut-off frequency at 0.15Hz) to remove noise and artifacts.
Dynamic FNC estimation
The dFNC of the whole brain was estimated via a sliding window approach, as shown in Fig. 2C (Step 1). We used a tapered window obtained by convolving a rectangle (window size = 20 TRs = 47.2 s) with a Gaussian (σ = 3) to localize the dataset at each time point. Prior research revealed that a window size between 30 and 60 s is a suitable option for capturing dFNC variation 77. Thus, we assumed that a window size of 47.2 s is a reasonable choice. Next, within each window, we employed Pearson correlation to assess the functional network connectivity among all 53 ICs within each window. Given the 53 ICs, this resulted in a symmetric 53×53 matrix. Furthermore, with these 53 ICs, we derived a total of connectivity variables for each window, encapsulating the comprehensive interconnections among the components. We then concatenated the dFNCs of each participant to form a (C × C × T) array (where C = 53 denotes the number of ICs and T = 210), which represented the changes in brain connectivity between ICs as a function of time 76.
Dynamic functional network connectivity clustering
We next concatenated the dFNC of all subjects and applied the k-means clustering algorithm to the dFNC windows to partition the data into sets of distinct clusters representing transient connectivity “states” (as shown in Step 2 of Fig.1C) 23–25,74. The optimal number of cluster order was estimated using the elbow criterion based on the ratio of within to between cluster distances. By sweeping the k-value from 2 to 9, we found that the optimal number of clusters was three 24. We used Euclidian distance as a distance metric in this k-means clustering algorithm with 1000 iterations. The k-means clustering analysis yielded three distinct states across all 275 participants and a state vector for each individual. The state vector reflects the temporal changes in whole-brain FNC. Subsequently, we determined the occupancy rate (OCR) for each participant, which is the proportion of time spent in each state. To compute the OCR for state i for a participant, we counted the number of windows in state i attributed to that participant and divided this by 210 (the total number of windows). Thus, we obtained three OCR values for each individual, corresponding to the three states. (Step 3 in Fig. 1C). Two representative state vectors of PTS and non-PTS individuals and their associated OCR for each state are shown in Supplementary Fig. 3.
Statistical analysis
We employed a General Linear Model (GLM) to explore the association between OCRs and PCL-5 scores using data from all participants (N=275). Our analysis included covariates such as age, sex at birth, years of education, income, employment status, marital status, scanning site, type of trauma, and percentile ADI. We constructed individual models for each OCR and time point, resulting in a total of 15 models derived from the combination of 3 predictors and 5 time points. Additionally, we developed 15 models for male group (N=94) and female group (N=181). In the context of sex-stratified analyses, sex itself was excluded as a covariate, and the analysis was run separately for each sex group. Therefore, we had 15 models for the whole group analysis, 15 models for the female group analysis, and 15 models for the male group analysis. A Benjamini-Hochberg false discovery rate correction was applied to account for the 15 significance tests corresponding to the correlations of each analysis 78 . In this study, all data analysis and statistical computations were conducted using MATLAB software (MathWorks, Natick, MA, USA) version R2022a.
Supplementary Material
Acknowledgements
The investigators express their gratitude to the trauma survivors participating in the AURORA study. Their time and effort during a challenging period of their lives enable our work to improve recovery outcomes for future trauma survivors. The AURORA project was supported by the National Institute of Mental Health (NIMH) under U01MH110925, the US Army MRMC, One Mind, and the Mayday Fund. The following authors received financial support from the AURORA project: R.C.K., K.J.R., K.C.K., S.A.M., S.L.H., F.L.B., X.A., J.S.S., T.C.N., G.D.C., T.J., S.D.L., L.T.G., K.A.B., S.L.R., J.P.H., A.B.S., C.L., P.I.M., P.L.H., S.S., C.W.J., B.E.P., R.A.S., V.P.M., J.L.P., M.J.S., E.H., C.P., D.A.P., R.C.M., R.M.D., N.K.R., B.J.O., L.D.S., S.E.B., J.J., D.A.P., J.F.S., and S.E.H. Additionally, this project received support from NIMH under T32MH125786 (M.S.E.S), Phyllis and Jerome Lyle Rappaport Mental Health Research Scholars Award (M.S.E.S), K01MH129828 (N.G.H.), K01MH121653 (S.J.H.v.R), R01MH129495 (T.J.), R01MH122867 (T.J.), R01MH121617 (L.T.G.), and R01MH123610 (V.D.C). The content is the sole responsibility of the authors and does not necessarily represent the official views of the funders. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation. Verily Life Sciences and Mindstrong Health provided some of the hardware and software for study assessments, and The Many Brains Project contributed software for neurocognitive assessments. This paper reflects the authors’ views and may not reflect the opinions or views of the NIH or the submitters of original data to the NIMH Data Archive (NDA).
Footnotes
Competing Interests Statement
Dr. Sendi has served as a consulatant for Niji Corp for unrelated work.
Dr. Daskalakis is on the scientific advisory board for Sentio Solutions, Inc. and Circular Genomics, Inc.
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka Pharmaceuticals, Sage Therapeutics, and Takeda Pharmaceuticals; honoraria from the Psychonomic Society and the American Psychological Association (for editorial work) and Alkermes, and research funding from the Bird Foundation, Brain and Behavior Research Foundation, DARPA, Millennium Pharmaceuticals, and the National Institute of Mental Health. In addition, he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), and Neuroscience Software.
Dr. Neylan has received research support from NIH, VA, and Rainwater Charitable Foundation, and consulting income from Jazz Pharmaceuticals.
In the last three years Dr Clifford has received research funding from the NSF, NIH and LifeBell AI, and unrestricted donations from AliveCor Inc, Amazon Research, the Center for Discovery, the Gates Foundation, Google, the Gordon and Betty Moore Foundation, MathWorks, Microsoft Research, Nextsense Inc, One Mind Foundation, and the Rett Research Foundation. Dr Clifford has financial interest in AliveCor Inc and Nextsense Inc. He also is the CTO of MindChild Medical with significant stock. These relationships are unconnected to the current work.
Dr. Germine is on the board of the Many Brains Project. Her family also has equity in Intelerad Medical Systems, Inc.
Dr. Rauch reported serving as secretary of the Society of Biological Psychiatry; serving as a board member of Community Psychiatry and Mindpath Health; serving as a board member of National Association of Behavioral Healthcare; serving as secretary and a board member for the Anxiety and Depression Association of America; serving as a board member of the National Network of Depression Centers; receiving royalties from Oxford University Press, American Psychiatric Publishing Inc, and Springer Publishing; and receiving personal fees from the Society of Biological Psychiatry, Community Psychiatry and Mindpath Health, and National Association of Behavioral Healthcare outside the submitted work.
Dr. Jones has no competing interests related to this work, though he has been an investigator on studies funded by AstraZeneca, Vapotherm, Abbott, and Ophirex.
Dr. Harte has no competing interest related to this work, though in the last three years he has received research funding from Aptinyx and Arbor Medical Innovations, and consulting payments from Aptinyx.
In the past 3 years, Dr. Kessler was a consultant for Cambridge Health Alliance, Canandaigua VA Medical Center, Holmusk, Partners Healthcare, Inc., RallyPoint Networks, Inc., and Sage Therapeutics. He has stock options in Cerebral Inc., Mirah, PYM, and Roga Sciences.
Dr. Koenen’s research has been supported by the Robert Wood Johnson Foundation, the Kaiser Family Foundation, the Harvard Center on the Developing Child, Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard, the National Institutes of Health, One Mind, the Anonymous Foundation, and Cohen Veterans Bioscience. She has been a paid consultant for Baker Hostetler, Discovery Vitality, and the Department of Justice. She has been a paid external reviewer for the Chan Zuckerberg Foundation, the University of Cape Town, and Capita Ireland. She has had paid speaking engagements in the last three years with the American Psychological Association, European Central Bank. Sigmund Freud University – Milan, Cambridge Health Alliance, and Coverys. She receives royalties from Guilford Press and Oxford University Press.
Dr. McLean has served as a consultant for Walter Reed Army Institute for Research, Arbor Medical Innovations, and BioXcel Therapeutics, Inc.
Dr. Ressler has performed scientific consultation for Bioxcel, Bionomics, Acer, and Jazz Pharma; serves on Scientific Advisory Boards for Sage, Boehringer Ingelheim, Senseye, and the Brain Research Foundation, and he has received sponsored research support from Alto Neuroscience.
The remaining authors declare no competing interests.
Data Availability
The data utilized in the preparation of this manuscript are publicly available in the National Institute of Mental Health (NIMH) Data Archive (NDA). The dataset identifier for this study is NIMH Data Archive Digital Object Identifier (DOI) 10.15154/zwyn-rb26.
Code Availability
The code used for preprocessing and dFNC calculation are available at https://trendscenter.org/software/. Also, statistical parametric mapping (SPM 12) is available at https://www.fil.ion.ucl.ac.uk/spm/.The Neuromark framework and the Neuromark template (Neuromark_fMRI_1.0) have been made available and incorporated into the Group ICA Toolbox (GIFT v4.0.5.14: https://trendscenter.org/software/gift/). Users worldwide can now directly download and utilize these resources. The chord graphs are generated using the NiChord toolbox in Python (https://github.com/paulcbogdan/NiChord). The General Linear Model (GLM) code in MATLAB is available in https://www.mathworks.com/help/stats/fitglm.html.
References
- 1.Pitman RK et al. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience vol. 13 769–787 Preprint at 10.1038/nrn3339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacella ML, Hruska B & Delahanty DL The physical health consequences of PTSD and PTSD symptoms: A meta-analytic review. Journal of Anxiety Disorders vol. 27 33–46 Preprint at 10.1016/j.janxdis.2012.08.004 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Schmidt U, Kaltwasser SF & Wotjak CT Biomarkers in posttraumatic stress disorder: Overview and implications for future research. Disease Markers vol. 35 43–54 Preprint at 10.1155/2013/835876 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michopoulos V, Norrholm SD & Jovanovic T Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biological Psychiatry vol. 78 344–353 Preprint at 10.1016/j.biopsych.2015.01.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Jowf GI, Ahmed ZT, Reijnders RA, de Nijs L & Eijssen LMT To Predict, Prevent, and Manage Post-Traumatic Stress Disorder (PTSD): A Review of Pathophysiology, Treatment, and Biomarkers. Int J Mol Sci 24, 5238 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roeckner AR, Oliver KI, Lebois LAM, van Rooij SJH & Stevens JS Neural contributors to trauma resilience: a review of longitudinal neuroimaging studies. Translational Psychiatry vol. 11 Preprint at 10.1038/s41398-021-01633-y (2021). [DOI] [Google Scholar]
- 7.Neria Y Functional neuroimaging in PTSD: From discovery of underlying mechanisms to addressing diagnostic heterogeneity. American Journal of Psychiatry vol. 178 128–135 Preprint at 10.1176/appi.ajp.2020.20121727 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Carrion VG, Wong SS & Kletter H Update on Neuroimaging and Cognitive Functioning in Maltreatment-Related Pediatric PTSD: Treatment Implications. J Fam Violence 28, 53–61 (2013). [Google Scholar]
- 9.Chen HJ et al. Altered resting-state dorsal anterior cingulate cortex functional connectivity in patients with post-traumatic stress disorder. Australian and New Zealand Journal of Psychiatry 53, 68–79 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Shaw ME et al. Abnormal functional connectivity in posttraumatic stress disorder. Neuroimage 15, 661–674 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Jin C et al. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol Med 44, 1927–1936 (2014). [DOI] [PubMed] [Google Scholar]
- 12.van Rooij SJH et al. The Role of the Hippocampus in Predicting Future Posttraumatic Stress Disorder Symptoms in Recently Traumatized Civilians. Biol Psychiatry 84, 106–115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens JS et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47, 1469–1478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breukelaar IA, Bryant RA & Korgaonkar MS The functional connectome in posttraumatic stress disorder. Neurobiol Stress 14, (2021). [Google Scholar]
- 15.Lebois LAM et al. Large-scale functional brain network architecture changes associated with trauma-related dissociation. American Journal of Psychiatry 178, 165–173 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett A et al. Longitudinal changes in brain function associated with symptom improvement in youth with PTSD. J Psychiatr Res 114, 161–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malivoire BL, Girard TA, Patel R & Monson CM Functional connectivity of hippocampal subregions in PTSD: Relations with symptoms. BMC Psychiatry 18, (2018). [Google Scholar]
- 18.Suo X et al. Individualized Prediction of PTSD Symptom Severity in Trauma Survivors From Whole-Brain Resting-State Functional Connectivity. Front Behav Neurosci 14, (2020). [Google Scholar]
- 19.Belleau EL et al. Amygdala functional connectivity in the acute aftermath of trauma prospectively predicts severity of posttraumatic stress symptoms: Functional connectivity predicts future PTSD symptoms. Neurobiol Stress 12, (2020). [Google Scholar]
- 20.Harnett NG et al. Prognostic neuroimaging biomarkers of trauma-related psychopathology: resting-state fMRI shortly after trauma predicts future PTSD and depression symptoms in the AURORA study. Neuropsychopharmacology 46, 1263–1271 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher J et al. Dynamic functional connectivity changes in dementia with Lewy bodies and Alzheimer’s disease. Neuroimage Clin 22, 101812 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabany L et al. Dynamic functional connectivity in schizophrenia and autism spectrum disorder: Convergence, divergence and classification. Neuroimage Clin 24, 101966 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sendi MSE et al. Aberrant Dynamic Functional Connectivity of Default Mode Network in Schizophrenia and Links to Symptom Severity. Front Neural Circuits 15, 1–14 (2021). [Google Scholar]
- 24.Dini H et al. Dynamic Functional Connectivity Predicts Treatment Response to Electroconvulsive Therapy in Major Depressive Disorder. Front Hum Neurosci 15, 1–11 (2021). [Google Scholar]
- 25.Sendi MSE et al. Aberrant dynamic functional connectivity of default mode network predicts symptom severity in major depressive disorder. Brain Connect (2021) doi: 10.1089/brain.2020.0748. [DOI] [Google Scholar]
- 26.Allen EA et al. Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex 24, 663–676 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preti MG, Bolton TA & Van De Ville D The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage 160, 41–54 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Calhoun VD, Miller R, Pearlson G & Adali T The Chronnectome: Time-Varying Connectivity Networks as the Next Frontier in fMRI Data Discovery. Neuron vol. 84 262–274 Preprint at 10.1016/j.neuron.2014.10.015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Z et al. Dynamic functional network connectivity associated with post-traumatic stress symptoms in COVID-19 survivors. Neurobiol Stress 15, (2021). [Google Scholar]
- 30.Dai Y et al. Altered dynamic functional connectivity associates with post-traumatic stress disorder. Brain Imaging Behav (2023) doi: 10.1007/s11682-023-00760-y. [DOI] [Google Scholar]
- 31.Wen Z, Seo J, Pace-Schott EF & Milad MR Abnormal dynamic functional connectivity during fear extinction learning in PTSD and anxiety disorders. Mol Psychiatry 27, 2216–2224 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu S et al. Altered local and large-scale dynamic functional connectivity variability in posttraumatic stress disorder: A resting-state fMRI study. Front Psychiatry 10, (2019). [Google Scholar]
- 33.Dobie DJ et al. Posttraumatic Stress Disorder in Female Veterans Association With Self-reported Health Problems and Functional Impairment. Arch Intern Med 164, 394–400 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Golin CE et al. Post-traumatic stress disorder symptoms and mental health over time among low-income women at increased risk of HIV in the U.S. J Health Care Poor Underserved 27, 891–910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rees SJ et al. A high-risk group of pregnant women with elevated levels of conflict-related trauma, intimate partner violence, symptoms of depression and other forms of mental distress in post-conflict Timor-Leste. Transl Psychiatry 6, (2016). [Google Scholar]
- 36.Rayburn NR et al. Trauma, depression, coping, and mental health service seeking among impoverished women. J Consult Clin Psychol 73, 667–677 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Webb EK et al. Neural impact of neighborhood socioeconomic disadvantage in traumatically injured adults. Neurobiol Stress 15, (2021). [Google Scholar]
- 38.McLean SA et al. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry 25, 283–296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bovin MJ et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess 28, 1379–1391 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Du Y, Fu Z & Calhoun VD Classification and prediction of brain disorders using functional connectivity: Promising but challenging. Frontiers in Neuroscience vol. 12 Preprint at 10.3389/fnins.2018.00525 (2018). [DOI] [Google Scholar]
- 41.Jin C et al. Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum Brain Mapp 38, 4479–4496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tozlu C, Jamison K, Gauthier SA & Kuceyeski A Dynamic Functional Connectivity Better Predicts Disability Than Structural and Static Functional Connectivity in People With Multiple Sclerosis. Front Neurosci 15, (2021). [Google Scholar]
- 43.Tejavibulya L et al. Predicting the future of neuroimaging predictive models in mental health. Molecular Psychiatry vol. 27 3129–3137 Preprint at 10.1038/s41380-022-01635-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertocci MA et al. Altered patterns of central executive, default mode and salience network activity and connectivity are associated with current and future depression risk in two independent young adult samples. Mol Psychiatry 28, 1046–1056 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sendi MSE et al. Alzheimer’s Disease Projection From Normal to Mild Dementia Reflected in Functional Network Connectivity: A Longitudinal Study. Front Neural Circuits 14, (2021). [Google Scholar]
- 46.MacGregor AJ, Joseph AR, Walker GJ & Dougherty AL Co-occurrence of hearing loss and posttraumatic stress disorder among injured military personnel: A retrospective study. BMC Public Health 20, (2020). [Google Scholar]
- 47.Mueller-Pfeiffer C et al. Atypical visual processing in posttraumatic stress disorder. Neuroimage Clin 3, 531–538 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rangaprakash D, Dretsch MN, Katz JS, Denney TS & Deshpande G Dynamics of segregation and integration in directional brain networks: Illustration in soldiers with PTSD and neurotrauma. Front Neurosci 13, (2019). [Google Scholar]
- 49.Lobo I et al. Hidden wounds of violence: Abnormal motor oscillatory brain activity is related to posttraumatic stress symptoms. Neuroimage 224, (2021). [Google Scholar]
- 50.Harnett NG et al. Structural covariance of the ventral visual stream predicts posttraumatic intrusion and nightmare symptoms: a multivariate data fusion analysis. Transl Psychiatry 12, (2022). [Google Scholar]
- 51.Harnett NG et al. Acute Posttraumatic Symptoms Are Associated With Multimodal Neuroimaging Structural Covariance Patterns: A Possible Role for the Neural Substrates of Visual Processing in Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 7, 129–138 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y et al. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J Affect Disord 187, 114–121 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Shang J et al. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: A resting-state fMRI study. PLoS One 9, (2014). [Google Scholar]
- 54.Avrahami D Visual art therapy’s unique contribution in the treatment of post-traumatic stress disorders. Journal of Trauma and Dissociation 6, 5–38 (2005). [Google Scholar]
- 55.Blithikioti C et al. The cerebellum and psychological trauma: A systematic review of neuroimaging studies. Neurobiology of Stress vol. 17 Preprint at 10.1016/j.ynstr.2022.100429 (2022). [DOI] [Google Scholar]
- 56.Baldaçara L et al. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J Psychiatr Res 45, 1627–1633 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Lebois LAM et al. Persistent Dissociation and Its Neural Correlates in Predicting Outcomes After Trauma Exposure. American Journal of Psychiatry 179, 661–672 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borst B et al. Sex differences in response inhibition-related neural predictors of PTSD in recent trauma-exposed civilians. Biol Psychiatry Cogn Neurosci Neuroimaging (2024) doi: 10.1016/j.bpsc.2024.03.002. [DOI] [Google Scholar]
- 59.Duncan LE et al. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry 23, 666–673 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nievergelt CM et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun 10, (2019). [Google Scholar]
- 61.Rowland GE et al. Prior Sexual Trauma Exposure Impacts Posttraumatic Dysfunction and Neural Circuitry Following a Recent Traumatic Event in the AURORA Study. Biological Psychiatry Global Open Science 3, 705–715 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill JL et al. A pilot study of closed-loop neuromodulation for treatment-resistant post-traumatic stress disorder. Nat Commun 14, (2023). [Google Scholar]
- 63.Zweerings J et al. Rt-fMRI neurofeedback-guided cognitive reappraisal training modulates amygdala responsivity in posttraumatic stress disorder. Neuroimage Clin 28, (2020). [Google Scholar]
- 64.Lieberman JM et al. Posterior cingulate cortex targeted real-time fMRI neurofeedback recalibrates functional connectivity with the amygdala, posterior insula, and default-mode network in PTSD. Brain Behav 13, (2023). [Google Scholar]
- 65.Bradley C, Nydam AS, Dux PE & Mattingley JB State-dependent effects of neural stimulation on brain function and cognition. Nature Reviews Neuroscience vol. 23 459–475 (2022). [DOI] [PubMed] [Google Scholar]
- 66.Lee SH & Dan Y Neuromodulation of Brain States. Neuron vol. 76 209–222 Preprint at 10.1016/j.neuron.2012.09.012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mccormick DA, Nestvogel DB & He BJ Annual Review of Neuroscience Neuromodulation of Brain State and Behavior. (2020) doi: 10.1146/annurev-neuro-100219. [DOI] [Google Scholar]
- 68.Sack AT et al. Target Engagement and Brain State Dependence of Transcranial Magnetic Stimulation: Implications for Clinical Practice. Biological Psychiatry vol. 95 536–544 Preprint at 10.1016/j.biopsych.2023.09.011 (2024). [DOI] [PubMed] [Google Scholar]
- 69.Monti RP et al. Real-time estimation of dynamic functional connectivity network. Hum Brain Mapp 38, 202–220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calhoun VD, Liu J & Adali T A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 45, (2009). [Google Scholar]
- 71.van Rooij SJH et al. Defining the r factor for post-trauma resilience and its neural predictors. Nature Mental Health (2024) doi: 10.1038/s44220-024-00242-0. [DOI] [Google Scholar]
- 72.Dennis EL et al. Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: results from the PGC-ENIGMA PTSD consortium. Mol Psychiatry 26, 4315–4330 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harnett NG et al. Structural inequities contribute to racial/ethnic differences in neurophysiological tone, but not threat reactivity, after trauma exposure. Mol Psychiatry (2023) doi: 10.1038/s41380-023-01971-x. [DOI] [Google Scholar]
- 74.Sendi MSE et al. The link between static and dynamic brain functional network connectivity and genetic risk of Alzheimer’s disease. Neuroimage Clin 37, (2023). [Google Scholar]
- 75.Du Y et al. NeuroMark: An automated and adaptive ICA based pipeline to identify reproducible fMRI markers of brain disorders. Neuroimage Clin 28, 102375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sendi MSE et al. Alzheimer’s Disease Projection From Normal to Mild Dementia Reflected in Functional Network Connectivity: A Longitudinal Study. Front Neural Circuits 14, (2021). [Google Scholar]
- 77.Yaesoubi M, Adalı T & Calhoun VD A window-less approach for capturing time-varying connectivity in fMRI data reveals the presence of states with variable rates of change. Hum Brain Mapp 39, 1626–1636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benjamini Yoav; Hochberg Yosef. Controlling the False Discovery Rate : A Practical and Powerful Approach to Multiple Testing. Royal Statistical Society . Series B ( Methodological ) 57, 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized in the preparation of this manuscript are publicly available in the National Institute of Mental Health (NIMH) Data Archive (NDA). The dataset identifier for this study is NIMH Data Archive Digital Object Identifier (DOI) 10.15154/zwyn-rb26.


