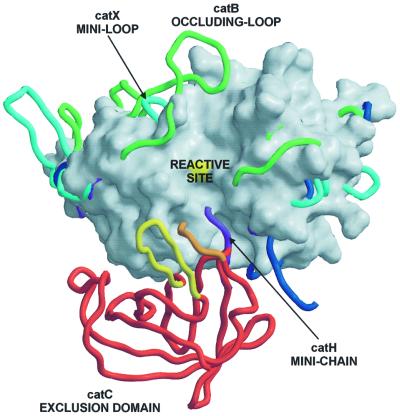
Fig. 4. Features of papain-like exopeptidases. A view towards the active site clefts of superimposed papain-like proteases. The underlying molecular surface of cathepsin L, shown in white, is used to demonstrate an endopeptidase active site cleft, which is blocked by features of the exopeptidase structures. The surface of the catalytic cysteine is colored in yellow. Chain traces of cathepsins B, X and H are shown in green, cyan and purple, respectively. Chain traces of papain-like domains of DPPI are shown in dark blue, whereas for the chain trace of the exclusion domain the color code is the same as in Figure 1. The bleomycin hydrolase chain trace is not shown for reasons of clarity, although its C-terminal residues superimpose almost perfectly with the C-terminal residues of the cathepsin H mini-chain (purple).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.