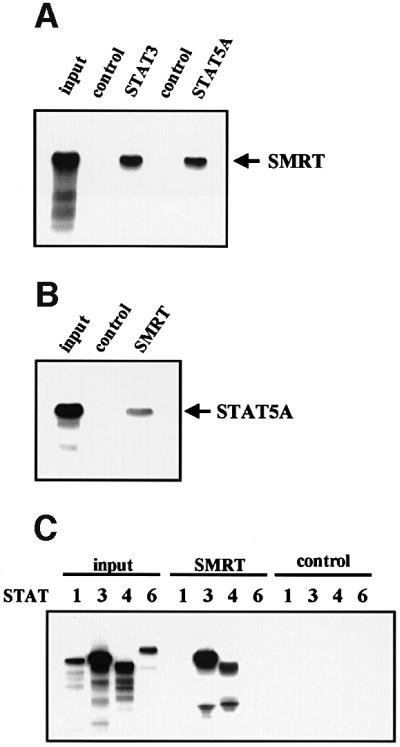
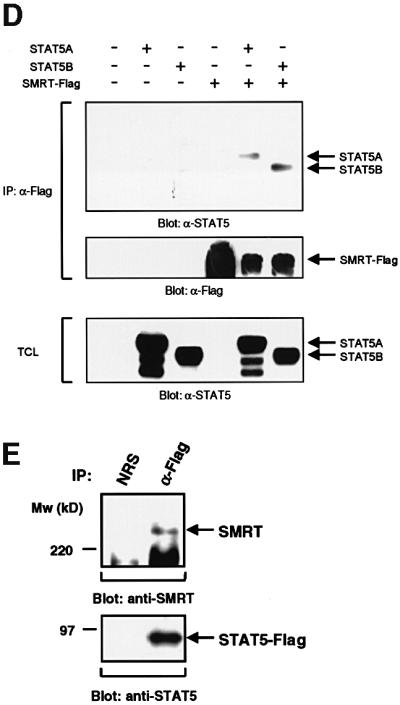
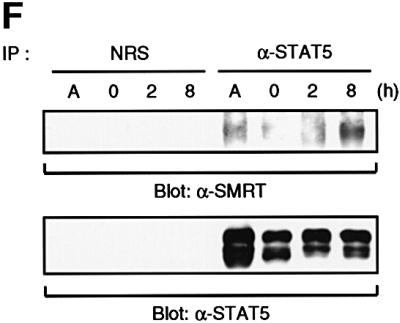
Fig. 2. Interaction of STAT5 and SMRT in vitro and in vivo. (A) In vitro translated [35S]methionine-labeled SMRT was incubated with STAT3 and STAT5A proteins immobilized on Sepharose beads in vitro. STAT3 and STAT5A proteins were generated in Sf9 cells as described previously (Quelle et al., 1996). Bound proteins were eluted and analyzed by SDS–PAGE. Input indicates 20% of 35S-labeled protein used in the reaction. Sepharose beads alone were used as a control. (B and C) In vitro translated [35S]methionine-labeled STAT1, 3, 4, 5A and 6 were incubated in vitro with SMRT-Flag protein immobilized on Sepharose beads. SMRT-Flag protein was expressed in Cos7 cells and immobilized on Sepharose beads by anti-Flag antibody. Bound proteins were eluted and analyzed on 4–20% SDS–PAGE gel. Input indicates 20% of 35S-labeled protein used in the reaction. Sepharose beads alone were used as a control. (D) In vivo interaction of STAT5 and SMRT. Extracts of 293 cells expressing SMRT-Flag and STAT5A or 5B were immunoprecipitated with anti-Flag antibody. Precipitated proteins were resolved on 4–20% SDS–PAGE gel, transferred to nitrocellulose membrane and immunoblotted with anti-STAT5 or anti-Flag monoclonal antibodies. Total cell lysates (TCL) were also run on 4–20% SDS–PAGE gel and immunoblotted with anti-STAT5 monoclonal antibody. (E) 32D cells expressing STAT5A-Flag were lysed for immunoprecipitation with non-immunized rabbit serum (NRS) or anti-Flag antibody. Precipitated proteins were eluted and analyzed by SDS–PAGE. Co-precipitated SMRT protein was detected by anti-SMRT polyclonal antibody. (F) The IL-3-dependent cell line DA3 was starved overnight and stimulated with 10 ng/ml IL-3 for the time period indicated. Cells were lysed, immunoprecipitated with NRS or anti-STAT5 antibody and analyzed by SDS–PAGE. A, asynchronously growing cells.
