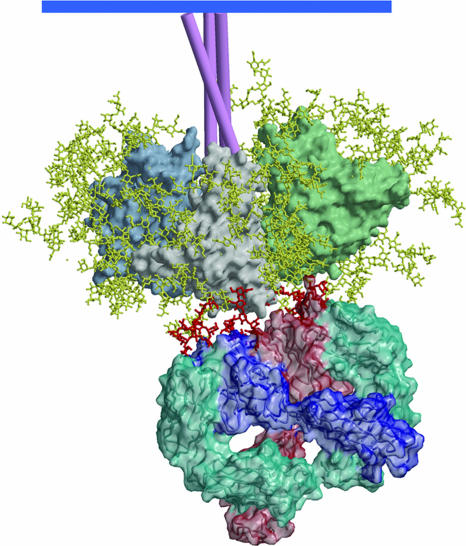
Fig. 5.
A model of mAb 2G12 Fab2 bound to the HIV-1 Env spike. The heavy chains of 2G12 are shown in dark blue and light red, and the light chains are shown in azure. The domain-swapped structure of 2G12 uncomplexed and complexed with Manα1–2Man and with Man9GlcNAc2 is described in ref. 46. The gp120 oligomannose residues important in 2G12 binding were assigned based on data from a number of different approaches (44, 45). Docking of the structure of 2G12, complexed in the conventional VH–VL combining sites with Man9GlcNAc2, onto gp120 places the GlcNac2 groups very close to N332 and N392 (outer dark red moieties). The Man9GlcNAc2 group attached to N339 (middle dark red) can be readily modeled to interact with the nonconventional VH–VH interface region.