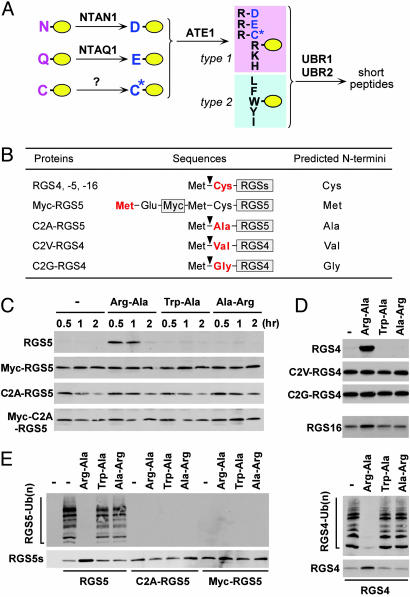
Fig. 1.
In vitro proteolysis of RGS4, RGS5, and RGS16. (A) The organization of mammalian N-end rule pathway, established from previous and current studies. N-terminal Asn and Gln function as pre-N-degrons through their deamidation by NTAN1 and a hypothetical enzyme, NTAQ1, respectively, into Asp and Glu (23). N-terminal Cys also serves as a pre-N-degron through its oxidation into Cys sulfinic acid (CysO2) or cysteic acid (CysO3) (ref. 3 and this study). N-terminal Asp, Glu, and (oxidized) Cys function as pre-N-degrons through their N-terminal arginylation by ATE1-encoded Arg-transferase (2). The resulting N-terminal Arg together with other type 1 and type 2 N-degrons are recognized by specific E3s for subsequent Ub-dependent proteolysis. We have previously characterized UBR1 and UBR2 as such E3s (14, 16). UBR1 and UBR2 are 200 kDa-RING finger E3s that target type 1 (Arg, Lys, and His) and type 2 (Phe, Leu, Trp, Tyr, and Ile) N-degrons (14, 16, 24). In mammals, N-terminal Ala, Ser, or Thr are classified as type 3 N-degron (16), but the E3 that recognizes type 3 N-degron remains elusive. (B) RGS constructs and their expected N-terminal residues. (C–D) RGS proteins were expressed in reticulocyte lysates either in the absence or presence of 2 mM dipeptides, and their degradation was analyzed by using time-course antibiotin Western blotting. Reactions were allowed for 60 min in D. Inhibition of RGS5 proteolysis by Arg-Ala diminished after 120 min perhaps because of instability of dipeptides. (E) RGS proteins were expressed in reticulocyte lysates in the presence of MG132. After 15 min, their ubiquitylation was analyzed by using anti-Ub immunoprecipitation and subsequent antibiotin Western blotting (Upper), while their expression levels were determined by using antibiotin Western blotting (Lower).