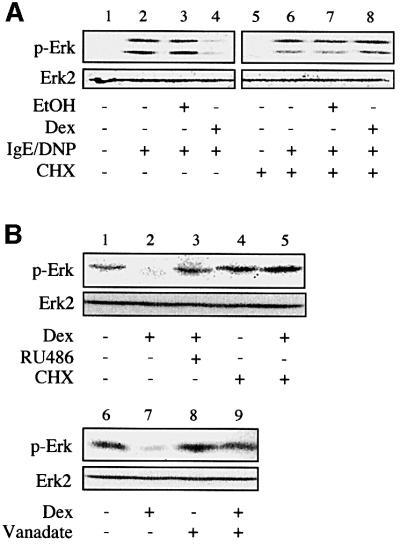
Fig. 2. Glucocorticoid-mediated inhibition of Erk-1/2 activation involves de novo expression of a tyrosine phosphatase. (A) RBL-2H3 mast cells were treated with dexamethasone (Dex, 0.1 µM) or solvent alone (EtOH) for 16 h in the presence or absence of cycloheximide (CHX), before sensitization with anti-DNP IgE, and activation with DNP-BSA. Phosphorylation of Erk-1/2 (p-Erk) was assessed by immunoblotting using a phospho-specific antibody. The membranes were stripped and reprobed with a phosphorylation state-independent anti-Erk-2 antibody. (B) Phosphorylated Erk-1/2 contained in lysates from IgE-sensitized and DNP-BSA-activated RBL-2H3 cells was subjected to a dephosphorylation assay. This was achieved by incubating the cellular extracts with lysates from RBL-2H3 cells treated for 16 h with dexamethasone (Dex, 0.1 µM) in the presence or absence of RU486 (1 µM) or cycloheximide (CHX). Incubation was performed in the absence (lanes 1–7) or the presence (lanes 8 and 9) of sodium orthovanadate (vanadate, 1 mM). The level of phosphorylation of Erk (p-Erk) was assessed by immunoblotting. The results are representative of three different experiments.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.