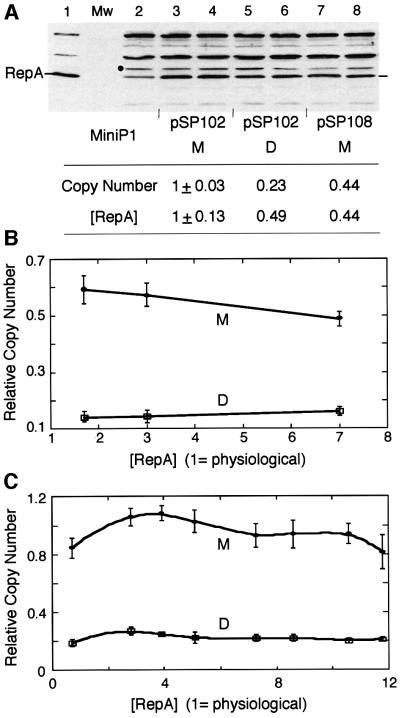
Fig. 2. Relationship of [RepA] and [origin] of miniP1 (pSP102) M and D. (A) [RepA] was determined by immunoblotting. In lane 1, cells with an overproducer of RepA source were used to locate RepA. In the next lane, protein molecular weight (Mw) standards were used that did not react with RepA antibody. In lane 2, cells do not have a RepA source but show a cross-reacting band of the size of RepA. The band was also seen with a different RepA antibody (gift of S.Wickner). The intensity of this band was subtracted from the bands in corresponding positions of lanes 3–8 for determination of relative [RepA]. A lower copy number derivative of pSP102 (pSP108, lanes 7–8; Pal et al., 1986) was used to test the effect of lowering the copy number on RepA production from a monomeric source. The black dot represents the band chosen for normalizing RepA content among different lanes. Duplicate samples were used in lanes 3–8 and their average RepA content is shown in the table below the figure. (B and C) Copy number of miniP1 M and D at increasing [RepA] in trans. In (B), the miniP1 plasmid was pVM11 which is deleted for its own repA gene. RepA was provided from compatible plasmids pALA69, pALA177 and pALA176 that constitutively produced 1.7×, 3× and 7× protein relative to the amount produced by wild-type P1 plasmid (physiological amount = 1) (Swack et al., 1987). In (C), the miniP1 plasmid was pSP302, which is identical to pSP102 except for the presence of a repA103(Am) mutation (Austin et al., 1985), and the host (MC4100) was non-suppressing (Sozhamannan and Chattoraj, 1993). RepA was supplied at varying concentrations using different inducer concentrations from pSP206, which contains repA under the control of the lac promoter (Pal and Chattoraj, 1988). Note that in both (B) and (C), the copy number difference is largely maintained irrespective of [RepA].

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.