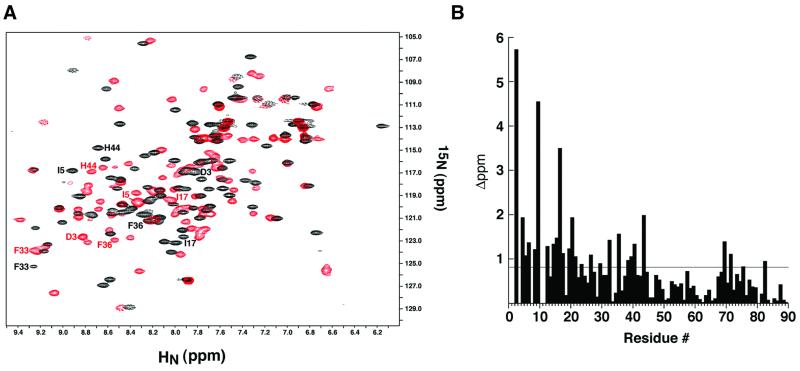
Fig. 3. Analysis of the σ537-binding surface by NMR ‘footprinting’. (A) AsiA homodimer (black) and the AsiA–σ537 complex (red) were analyzed by 15N–1H HSQC spectroscopy with 15N-labeled AsiA and unlabeled σ537. The positions of several residues that move substantially upon addition of σ537 are shown in black for the AsiA homodimer and in red for the AsiA–σ537 complex. (B) A histogram of the composite chemical shift changes (see Materials and methods) defines a broad ‘footprint’ (>0.8 p.p.m. change) of σ537 on AsiA that encompasses many of residues 3–20 and residues 33, 36, 39–40 and 44. These residues reside along the homodimer interface of AsiA.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.