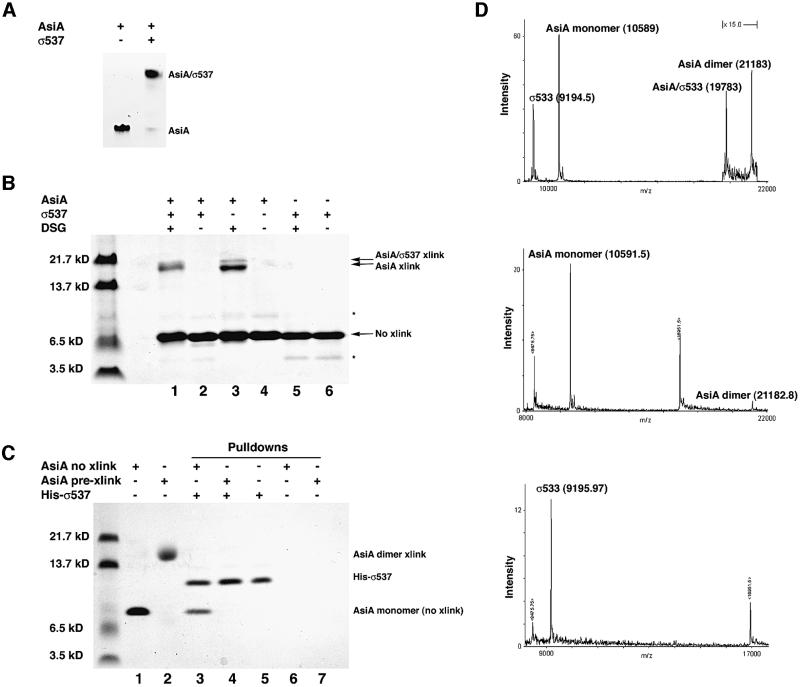
Fig. 4. AsiA forms a 1:1 complex with SR4. (A) Non-denaturing Tris-alanine PhastGel (Amersham/Pharmacia) at pH 8.8 demonstrating the homogeneity of the AsiA homodimer and AsiA–σ537 complex preparations used for protein cross-linking with DSG. σ537 fails to run into this gel matrix. (B) Denaturing SDS-tricine PAGE of homodimers and AsiA–σ537 complexes following cross-linking with DSG. σ537 fails to form a cross-link with DSG (lanes 5 and 6), in contrast to the AsiA homodimer (lanes 3 and 4) and the AsiA–σ537 complex (lanes 1 and 2). Cross-linking with the AsiA homodimer produces a major and minor species (lane 3), while cross-linking the heterodimer produces a single species of slightly different mobility with apparently the same size as the cross-linked AsiA homodimer. Note that non-denaturing PAGE (A) establishes that the cross-linked species in each case are either pure homodimer (lane 3) or pure complex (lane 1). (C) Pre-cross-linked AsiA does not bind σ537 in an affinity tag pull-down experiment. Solutions of purified AsiA (lane 1) or purified AsiA cross-linked with DSG (lane 2) were probed with N-terminal His6-tagged σ537 immobilized on Ni2+-Sepharose. While uncross-linked AsiA was pulled down by His-σ537 (lane 3), pre-cross-linked AsiA was no longer capable of binding His-σ537 (lane 4). This indicates that an AsiA dimer which cannot dissociate is unable to bind σ537 in solution. Lanes 6 and 7 indicate that neither uncross-linked AsiA (lane 6) nor pre-cross-linked AsiA (lane 7) bind to Ni2+-Sepharose on their own. (D) MALDI of AsiA, σ533 and AsiA–σ533 complexes (see Materials and methods for details). The mass spectra of the AsiA homodimer and σ533 are shown alone in the middle and bottom panels, respectively. The peaks bracketing the main peak are those of the myoglobin calibrant with the +1 ion at 16 951.5 and the +2 ion at 8475.75. The top panel shows the mass spectrum of the AsiA–σ533 mixture with AsiA homodimer. In this spectrum, the masses of both protein complexes are seen, along with each of the monomer species from which they are derived. This demonstrates that the mass of the AsiA–σ533 complex is that of a 1:1 complex with complete subunit dissociation from homodimer to heterodimer.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.