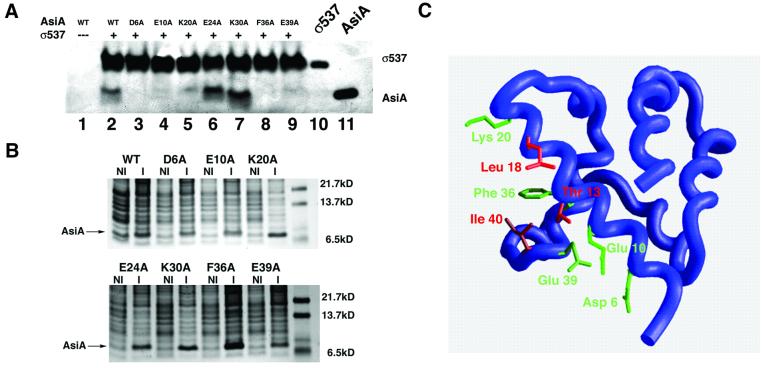
Fig. 6. Mutagenesis of the AsiA homodimer interface. (A) The indicated mutant proteins (lanes 2–9) were analyzed for their ability to bind σ537 immobilized on chelating Sepharose 4B. Lane 1 represents a pull-down assay with beads alone (i.e. Ni2+ beads lacking σ537). Lane 10 represents purified σ537 that has not been exposed to AsiA. Lane 11 is purified wild-type AsiA that has not been exposed to σ537. Alanine substitution of charged residues that reside at the AsiA homodimer interface (Asp6, Glu10 and Glu39) results in mutant proteins that fail to bind σ537. Alanine substitutions at two other residues, Glu24 and Lys30, retain the ability to bind AsiA in this assay. (B) Expression of wild-type and mutant AsiAs. Uninduced (NI) and induced (I) expression of wild-type and mutant AsiAs visualized by Coomassie Blue staining. The position of the induced band representing the wild-type or mutant proteins is indicated. (C) Mapping of the heterodimer interface onto the AsiA monomer structure. Red residues (Thr13, Leu18, Ala35 and Ile40) display intermolecular NOEs to SR4 in an AsiA–σ537 complex. Green residues (Asp6, Glu10, Lys20, Phe36 and Glu39) fail to bind SR4 in a pull-down assay when mutated to alanine. Ala35 is obscured in this view and cannot be seen.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.