Abstract
Myogenic differentiation can be initiated by a limited number of molecules. In this work, we analyzed the function of the homeobox gene Lbx1 in chicken embryos and explant cultures. We demonstrate that overexpression of Lbx1 in vivo and in vitro leads to a strong activation of various muscle markers. We show that cell proliferation, which is strongly stimulated by Lbx1 and Pax3, is required for Lbx1- or Pax3-dependent myogenic activation. Inhibition of cell proliferation prevents expression of muscle differentiation markers, while the activation of other putative downstream targets of Pax3 and Lbx1 is not affected. Our findings imply that a critical function of Pax3 and Lbx1 during muscle cell formation is the enlargement of muscle cell populations. The growth of the muscle precursor cell population may increase the bias for myogenic differentiation and thus enable myogenic cells to respond to environmental cues.
Keywords: homeobox gene/Lbx1/myogenesis/Pax3
Introduction
The majority of skeletal muscles are derived from transient structures of the mesoderm: the somites (Arnold and Braun, 2000). Somites can be subdivided into ventral and dorsal halves. Cells of the ventral half delaminate from the epithelial somite and form the sclerotome, which gives rise to the axial skeleton and ribs. The dorsal part of the somite remains epithelialized and forms the dermomyotome. Cells of the dermomyotome, which proliferate at a high rate, are the source of all epaxial and hypaxial muscle cells (Christ et al., 1977).
In contrast to the cells of the ventro-medial somite, where all the cells rapidly delaminate and are committed to the sclerotomal fate, muscle precursor cells are con tinuously recruited from the dorsal part of somite over many days. These muscle precursor cells are highly proliferative and responsive to permissive and instructive environmental cues. Although numerous studies have identified signals that emanate from the axial notochord, the neural tube (NT), the overlying lateral ectoderm and the lateral plate mesoderm (LPM), the exact nature and composition of these cues are still elusive.
A number of observations have been made that are not compatible with the view that instructive signals emanating from surrounding tissues at this relatively late stage of development determine the fate of muscle cells in the myotome and limb buds. Rather, they argue for a permissive role of signals that may act on cells that are already partially or completely committed to myogenesis before or during gastrulation. Such signals, which are generated during somitogenesis, might relieve the repression of muscle precursor cells imposed by surrounding tissues or cells (Cossu et al., 1996; George-Weinstein et al., 1996; Pourquie et al., 1996).
Another critical value in this context might be the control of cell proliferation and the resulting size of a potential ‘colony’ of muscle-forming cells. Gurdon has shown that a potential muscle cell must be within a group of cells of minimum size in order to respond to inductive signals or to differentiate autonomously. This cellular behavior, coined ‘community effect’ (Gurdon, 1988), was mainly based on transplantation experiments in Xenopus embryos where single cells failed to differentiate into muscle cells after transplantation, while groups of cells readily underwent myogenic differentiation. Interestingly, a few scattered Myf5-positive cells are present in NT, but normally do not undergo myogenic differentiation. However, when placed in culture, a small fraction of these cells can co-express muscle myosin heavy chain and neuronal β III tubulin within the same cell (Tajbakhsh et al., 1994).
Within the somite, two different myogenic cell populations have been identified by various means (Ordahl and Le Douarin, 1992). One cell population resides in the medial dermomyotome and generates epaxial muscles (such as intrinsic back muscles); the other originates from the lateral dermomyotome and produces hypaxial muscles of the body wall and limbs. Pax3, which is initially expressed throughout the entire paraxial mesoderm and later becomes restricted to the dermomyotome and hypaxial muscle precursor cells, is considered to be on top of the regulatory cascade leading to hypaxial muscle formation. Mutations in the Pax3 locus in splotch mice result in an absence or reduction of all hypaxial muscles (Bober et al., 1994). Hypaxial muscle development is also impaired in mice lacking Lbx1. Targeted deletion of Lbx1 leads to a failure of muscle precursor cells of the hindlimb and of extensor muscle precursor cells of the forelimb to migrate to their targets (Schäfer and Braun, 1999). In splotch mice, no Lbx1 expression is detectable in somite or limb buds (Mennerich et al., 1998), while Pax3 expression is normal in the remaining hypaxial muscle cells of Lbx1 mutant mice.
Beside its role in hypaxial muscle development, Pax3 appears to have a general function in muscle development. Generation of Pax3–Myf5 double-mutant mice yielded mice without body muscles (Tajbakhsh et al., 1997), and ectopic expression of Pax3 activates MyoD and Myf5 in embryonic mesoderm and neural tissue (Maroto et al., 1997). These results imply that Pax3 directly activates MyoD expression, while Myf5 is located in a different myogenic pathway. However, alternative explanations are feasible and a detailed model for this hypothesis is required.
In this work, we investigate the function of the transcription factors Lbx1 and Pax3 during myogenic differentiation in chicken embryos and explant cultures. We demonstrate that ectopic expression of Lbx1 in ovo leads to a strong activation of muscle cell formation in somites and limbs, but not in other ectopic locations. Ectopic expression of Lbx1 in explant cultures derived from several tissues induces various muscle cell markers. We show that cell proliferation, which is strongly stimulated both by Lbx1 and Pax3, is required for Lbx1- or Pax3-dependent myogenic activation. Inhibition of cell proliferation abrogates activation of the myogenic program, while the regulatory feedback loop initiated by Pax3 and Lbx1 is still active. Our results might provide an explanation of how Pax3 and Lbx1 induce myogenesis by amplification of the myogenic precursor cell pool with an increasing potential for myogenic differentiation, and emphasize the critical role of the size of cell populations biased for differentiation.
Results
Ectopic expression of Lbx1 enhances the expression of the myogenic factors MyoD and myogenin
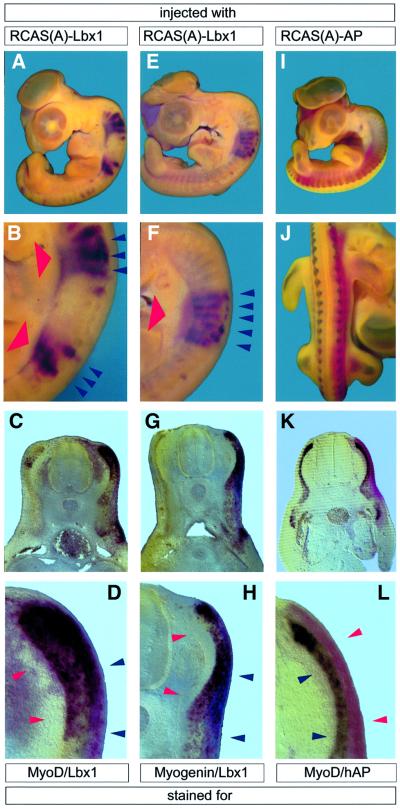
To analyze the effects of Lbx1 on myogenic differentiation, a RCAS(A)-based Lbx1 retrovirus was injected into somites I–IV or VI–X of HH10 chicken embryos. Three days after retroviral infection, at HH25, embryos were fixed and subjected to whole-mount in situ hybridization (Figure 1). The contralateral uninfected half of the embryo served as a negative control. As expected, the retrovirus generated a high level of expression of Lbx1 mRNA. No differences in expression levels of ectopic Lbx1 were discernible within infected tissues (red staining in Figure 1A–F). Likewise, a control virus encoding human alkaline phosphatase (hAP) yielded homogenously infected tissue, as indicated by staining for the enzymatic activity of the ectopically expressed hAP enzyme (red staining in Figure 1I–L).
Fig. 1. Forced expression of Lbx1 stimulates myogenesis in the paraxial segmented mesoderm in vivo. HH25 chicken embryos were subjected to whole-mount in situ hybridization after injection of RCAS(A)–Lbx1 (A–H) and RCAS(A)–hAP (I–L) into somites I–IV or VI–X of HH10 embryos and 3 days of incubation. Depending on the injection procedure, which involved several injections per embryo, either two separate sets (A and B), a single block of somites (E and F) or most somites of one half (I and J) were infected. Two-color whole-mount in situ hybridization of MyoD (dark purple staining in A–D) and Lbx1 (red staining in A–H) and of myogenin (dark purple staining in E–H) and Lbx1. Embryos in I–L were hybridized with a MyoD antisense probe (dark purple staining) and stained for hAP activity (red staining). Whole-mount preparations (A, B, E, F, I and J) and vibratome sections (C, D, G, H, K and L) are shown. Forced expression of Lbx1 (indicated by bold red arrowheads) resulted in a strong up-regulation of MyoD (A–D) and myogenin (E–H) in somites (blue arrowheads in B, D, F and H), while the expression of hAP did not result in an up-regulation of MyoD (I–L) and other myogenic markers. A shorter staining time in RCAS(A)–Lbx1-injected embryos allowed only the detection of stimulated MyoD and myogenin expression, while uninjected halves showed only a very weak MyoD (C) and myogenin (G) signal.
At the same time, myogenesis was evaluated in injected embryos by monitoring expression of MyoD and myogenin using two-color whole-mount in situ hybridization (dark stainings in Figure 1). Infection of paraxial mesoderm at varying axial positions led to high level expression of MyoD (Figure 1A–D) and myogenin (Figure 1E–H). Interestingly, enhanced expression was restricted to the segmented mesoderm. The segmental upregulation was detected for both MyoD (Figure 1A–D) and myogenin (Figure 1E–H). No significant activation of expression was found outside the somites, although the areas of enhanced expression did not completely co-localize with the endogenous expression pattern of these genes. This is particulary evident in Figure 1D and H, where MyoD and myogenin expression is present in the dermomyotome, which does not normally express either of these molecules. No up-regulation of MyoD or myogenin was observed in areas without forced expression of Lbx1.
In contrast, virus-mediated expression of hAP did not lead to up-regulation of MyoD (Figure 1I–L) or myogenin (not shown) in the myotome or ectopic expression in the dermomyotome. Only the normal expression pattern and signal strength of MyoD within the myotome were visible (Figure 1I–L).
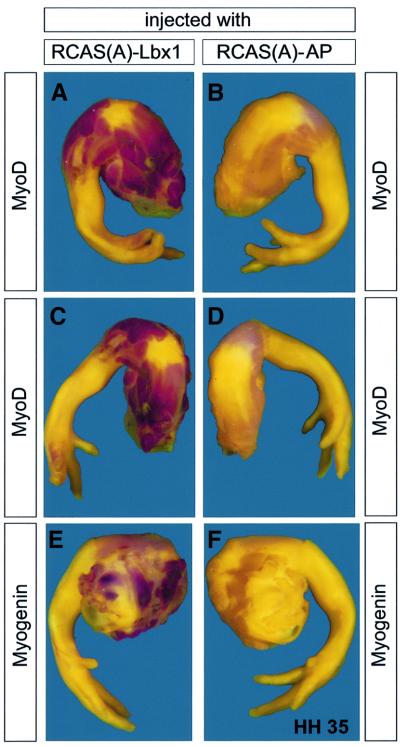
Lbx1 is mainly expressed in hypaxial muscle precursor cells that give rise to limb muscles. We therefore injected the Lbx1 retrovirus into leg buds of HH24 embryos and stained for an enhanced or ectopic expression of myogenin and MyoD at HH35 4 days later using whole-mount in situ hybridization. Although the size and density of the leg tissue prevented a complete penetration of the probe, we detected a striking up-regulation of MyoD (Figure 2A and C) and myogenin (Figure 2E) in prospective muscle tissue throughout the legs. No ectopic expression of MyoD and myogenin was found in epidermis and cartilage in these tissues. Similar to the situation in somites, we did not observe an enhancement of MyoD and myogenin expression when a RCAS(A)–hAP retrovirus (Figure 2B, D and F) or Pax3-encoding retrovirus was used (data not shown).
Fig. 2. Forced expression of Lbx1 stimulates myogenesis in the leg in vivo. HH35 chicken embryo wings were subjected to whole-mount in situ hybridization after injection of RCAS(A)–Lbx1 (A, C and E) and RCAS(A)–hAP (B, D and F) into leg buds of HH24 embryos and 4 days of incubation. Whole-mount in situ hybridizations with MyoD (red staining in A–D) and myogenin probes are shown (E and F). Forced expression of Lbx1 resulted in a strong up-regulation of MyoD (A and C) and myogenin (E) in presumptive muscles of the legs, while the expression of hAP did not result in an up-regulation of MyoD (B and D) and myogenin (F). Staining reactions for MyoD and myogenin were allowed to develop longer in RCAS(A)–hAP-injected embryos (B, D and F) to allow visualization of unstimulated MyoD and myogenin in the legs.
While the activation of ectopic and enhanced expression of myogenic factors was consistently observed in a large number of injected embryos (n = 42), we were never able to obtain similar effects with a Pax3-expressing retrovirus (n = 49). This inability of Pax3 to ectopically induce myogenesis in ovo in the context of an intact embryo is in full agreement with previous reports by Maroto et al. (1997) and Heanue et al. (1999).
Expression of Lbx1 or Pax3 activates myogenesis in embryonic mesoderm in the absence of inducing tissues and in neural tube explants
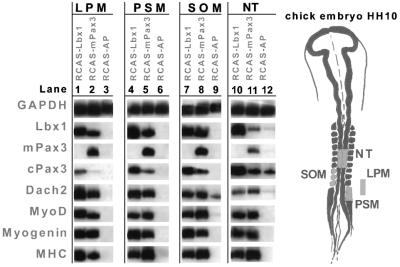
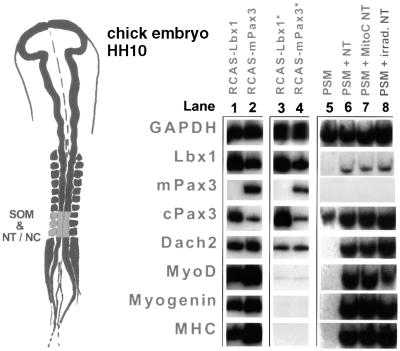
In vivo inducing and repressing signals act together to ensure that initiation of myogenesis occurs at a defined location within the embryo. In order to analyze whether forced expression of Lbx1 and Pax3 activated myogenesis in mesodermal fragments cultured in the absence of inducing or repressing tissues, we isolated three different mesodermal regions [paraxial pre-segmented mesoderm (PSM), lateral plate mesoderm (LPM) and somites] from chicken embryos at HH10 (Figure 3). Explants were infected with retroviruses encoding Lbx1, Pax3 or hAP, cultured in a collagen matrix for 5 days and analyzed by RT–PCR for expression of the myogenic markers MyoD, myogenin and embMyHC. Infection of all three types of mesodermal tissues with RCAS–mPax3 led to high level expression of MyoD, myogenin and embMyHC (Maroto et al., 1997) as well as to activation of Dach2 (Heanue et al., 1999) and Lbx1 mRNA (Figure 3, lanes 2, 5 and 8). In contrast, a control infection with RCAS–hAP did not result in an up-regulation of any of these markers (Figure 3, lanes 3, 6 and 9). Uninfected and mock-infected somites, which were cultured in the absence of muscle-inducing tissues, lost expression of Pax3 within 12 h and failed to undergo myogenic differentiation (Figure 3). Likewise, expression of Pax3 in uninfected and mock-infected PSM was not sustained in the absence of muscle-inducing tissues and myogenesis did not occur (Figure 3).
Fig. 3. Forced Lbx1 and Pax3 expression induces myogenesis in various parts of the mesoderm and NT. Tissues [LPM, lanes 1–3; PSM, lanes 4–6; somites (SOM), lanes 7–9; NT, lanes 10–12] were dissected from HH10 embryos (indicated by the yellow color), separated from surrounding tissues and cultured in collagen gels. RNA was isolated 5 days after infection with RCAS(A)–Lbx1 (lanes 1, 4, 7 and 10), RCAS(A)–Pax3 (lanes 2, 5, 8 and 11) and RCAS(A)–hAP (lanes 3, 6, 9 and 12), and analyzed by RT–PCR using various primer pairs. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MHC, embryonic myosin heavy chain. Exogenous expression of Pax3 was monitored using primers specific for the mouse cDNA (mPAX3). Both Lbx1 and Pax3, but not the control virus, were able to induce myogenic markers including MHC. Low level endogenous Dach2 expression was detectable in isolated SOM (lane 9), but strongly up-regulated by Lbx1 (lane 7) and Pax3 (lane 8).
Infection of somites, PSM and LPM with RCAS–Lbx1 resulted in a strong activation of the myogenic markers MyoD, myogenin and embMyHC as well as of Pax3 and Dach2 (Figure 3, lanes 1, 4 and 7). It is interesting to note that the forced expression of Lbx1 yielded a robust up-regulation of Pax3 in all three mesodermal tissue fragments despite the fact that Pax3 is genetically upstream of Lbx1 (Mennerich et al., 1998).
It has been reasoned that either the lack of a co-factor or the presence of a repressor might be responsible for the lack of myogenesis in the neural tube, which can be overcome by forced expression of Pax3 (Maroto et al., 1997). To test whether Lbx1 is capable of generating signals sufficient to alleviate the repression of muscle cell formation in this tissue, fragments of NT, isolated at the level of somites IV–VI from HH10 embryos, were infected with RCAS–Lbx1, RCAS–Pax3 and RCAS–hAP. Analy sis of myogenic regulators such as MyoD, myogenin and Dach2, as well as terminal differentiation markers such as embMyHC, revealed a strong initiation of myogenesis (Figure 3, lanes 10 and 11). Owing to the endogenous expression of Lbx1, Pax3 and Dach2 mRNAs in NT, which were not lost in explanted NT fragments as in embryonic mesoderm separated from surrounding tissues, basal levels of these molecules were found in RCAS–hAP and uninfected tissue (Figure 3, lane 12). Dach2 was up-regulated above the basal level by RCAS–Lbx1 and RCAS–Pax3. Pax3 expression was virtually unaffected by its own forced expression, but strongly induced by RCAS–Lbx1. Likewise, Lbx1 was induced by Pax3 in NT.
Lbx1, Pax3 and Dach2 participate in a positive regulatory feedback loop to activate the myogenic differentiation program
The partially overlapping expression pattern of Lbx1, Pax3 and Dach2 and the analysis of Pax3 and Lbx1 mutant mice suggested that these molecules might act in a common regulatory pathway (Bober et al., 1994; Mennerich et al., 1998; Heanue et al., 1999; Schäfer and Braun, 1999). In addition, it has been shown that Pax3 and Dach2 positively regulate each other in tissue culture explants (Heanue et al., 1999). To analyze whether Pax3, Lbx1 and Dach2 participate in a positive regulatory loop in various cellular backgrounds, we expressed Lbx1 and Pax3 in chicken dermal fibroblasts and murine 10T1/2 fibroblasts. Infection of chicken dermal fibroblasts with RCAS–Lbx1 induced expression of Pax3, Dach2, MyoD and MyHC. In contrast, transfection of murine 10T1/2 fibroblasts with an Lbx1 expression plasmid did not result in enhanced expression of Pax3, Dach2, MyoD and MyHC (Figure 4, lanes 1 and 5). Similarly, Lbx1, Dach2, MyoD, MyHC and the endogenous Pax3 gene were induced after infection of chicken dermal fibroblasts with RCAS–Pax3, while expression of Pax3 in murine 10T1/2 fibroblasts by a cytomegalovirus (CMV) promotor-driven expression plasmid did not generate a detectable expression level of these genes (Figure 4, lanes 3 and 7). Similar expression levels of the ectopically expressed genes were reached in chicken dermal fibroblasts and 10T1/2 fibroblasts irrespective of the use of viral or plasmid-based expression systems (Figure 4, lanes 3 and 7), ruling out the possibility that the different cellular response is due to a different concentration of exogenous Lbx1 and Pax3 within the cells. As expected, infection of dermal fibroblasts with a control RCAS–hAP retrovirus did not initiate myogenesis (Figure 4, lanes 2 and 4).
Fig. 4. Activation of myogenesis by forced expression of Lbx1 and Pax3 depends on the cell type. Lbx1 (lanes 1 and 5), hAP (lanes 2, 4, 6 and 8) and Pax3 (lanes 3 and 7) were expressed in primary chicken cells (lanes 1–4) and 10T1/2 mouse fibroblasts (lanes 5–8) using either a RCAS-based retrovirus (lanes 1–4) or a CMV promoter-based expression construct (lanes 5–8). After 3 days in differentiation medium, RNA was isolated and RT–PCR analysis was performed using primers specific for the chicken and the mouse mRNAs, respectively. Components of the Pax3–Lbx1–Dach2 regulatory loop as well as MyoD and MyHC were readily activated in primary chicken cells by Lbx1 (lane 1) and Pax3 (lane 3). In contrast, forced expression of Lbx1 or Pax3 did not result in activation of Pax3, Dach2 and endogenous Lbx1 or of MyoD and MyHC in 10T1/2 cells (lane 5) despite a strong expression of exogenous cLbx1 or mPax3 in these cells.
Use of the murine Pax3 cDNA allowed us to detect an up-regulation of the endogenous cPax3 gene. Since Pax3 also induces Lbx1 and Dach2, it remains unclear whether Pax3 acts in an auto-regulatory loop to stimulate its own expression or whether Lbx1 and Dach2 mediate this activation.
In conclusion, our experiments demonstrate that a positive regulatory feedback loop operates between Lbx1, Pax3 and Dach2 in certain cell types, such as primary dermal fibroblasts, while other cells like 10T1/2 fibroblasts are refractory to this regulatory loop.
Lbx1 and Pax3 enhance cell proliferation in embryonic mesoderm and NT explants
Lbx1 and Pax3 may directly regulate the transcription of myogenic regulatory genes, thereby inducing myogenesis. Alternatively, the role of Lbx1 and Pax3 in muscle cell formation might be more complicated, involving regulation of cell proliferation and generation of a commitment for myogenesis. To gain insight into the cellular mechanisms initiated by Lbx1 and Pax3, we investigated whether one or both genes may directly stimulate cell proliferation.
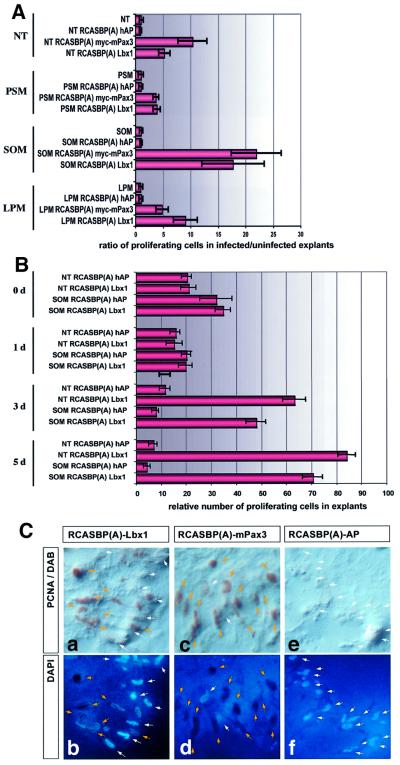
Tissue fragments derived from NT, unsegmented PSM, LPM and somites were infected with RCAS–Lbx1, RCAS–Pax3 and RCAS–hAP. The rate of cell proliferation within explants was determined after 5 days using an antibody against proliferating cell nuclear antigen (PCNA) that marks cells undergoing mitosis. Forced expression of Lbx1 and Pax3 generated a strong increase in the rate of cell proliferation compared with the RCAS–hAP control virus in all tissues tested (Figure 5A). The strongest increase was observed in explanted somites infected with RCAS–Pax3 (n = 5) and RCAS–Lbx1 (n = 8), where the ratio of proliferating cells compared with RCAS(A)–hAP and uninfected controls was raised 18-fold for Lbx1 and 22-fold for Pax3. In other tissues, the increase was less dramatic but still significant; in NT explants, Pax3 increased the proliferation rate 10.5-fold and Lbx1 5-fold. In LPM, the situation was nearly reversed; a 9-fold increase in the proliferation rate was detected after infection with RCAS(A)–Lbx1 and a 5-fold increase after infection with RCAS(A)–Pax3. The weakest increase was found in PSM, with an ∼4-fold increase for both Lbx1 and Pax3. Although Pax3 generated the strongest response in somites, Lbx1 is not necessarily less effective at stimulating cell proliferation, as clearly indicated by infection of the LPM, which repetitively gave stronger responses for Lbx1 (n = 8) than for Pax3 (n = 9). We also investigated the number of proliferating cells at various time points after infection with RCAS–Lbx1. As shown in Figure 5B, the number of proliferating cells was relatively high immediately after explantation (day 0). After infection with RCAS–Lbx1 and RCAS–hAP at day 0, the number initially fell (day 1). In cultures infected with the RCAS–hAP control virus, the reduction of proliferating cells continued during the course of the experiment. In contrast, we observed a strong increase in proliferating cells at day 3 and day 5 in cultures infected with RCAS–Lbx1. The number of proliferating cells clearly exceeded the amount of dividing cells that was initially present in explants, suggesting that Lbx1 recruits resting cells into the cell cycle. Although we did not trace the cells, which were induced to divide, the parallel increase in the expression of myogenic markers in infected cultures allows the conclusion that Lbx1 and Pax3 may increase the pool of muscle precursor cells and at the same time preserve or even improve their potential for myogenic differentiation.
Fig. 5. Forced expression of Lbx1 and Pax3 strongly stimulates cell proliferation in embryonic mesoderm and NT explants. (A) Tissue fragments derived from NT (columns 1–4), PSM (columns 5–8), SOM (columns 9–12) and LPM (columns 13–16) were infected with RCAS–Lbx1, RCAS–Pax3 and RCAS–hAP viruses. Proliferating cells were identified by staining for PCNA antigen. Values on the x-axis indicate x-fold induction of the number of proliferating cells in infected versus uninfected explants. (B) Tissue fragments derived from SOM or NT were infected with either RCAS–hAP or RCAS–Lbx1 and analyzed for the presence of proliferating cells at different time points after explantation. Stimulation of proliferation by Lbx1 became apparent 3 days after infection and exceeded the relatively high proliferation rate in cultures without Lbx1 at day 0. Values on the x-axis indicate the number of proliferating cells relative to the absolute number of cells in explants. (C) Representative example for Lbx1- and Pax3-induced cell proliferation. SOM explants were immunostained with a PCNA antibody (a, c and e) 5 days after infection with RCAS–Lbx1 (a and b), RCAS–Pax3 (c and d) and RCAS–hAP (e and f) viruses. Nuclear DNA was visualized by DAPI staining (b, d and f). Yellow arrows mark PCNA-positive cells; white arrows mark non-proliferating cells. In explants infected with RCAS–hAP, very few proliferating cells were seen, and in RCAS–Lbx1 and RCAS–Pax3 infected explants numerous PCNA-positive, proliferating cells were detectable.
Cell proliferation is required for Lbx1- and Pax3-mediated activation of myogenesis but not for Dach2 activation
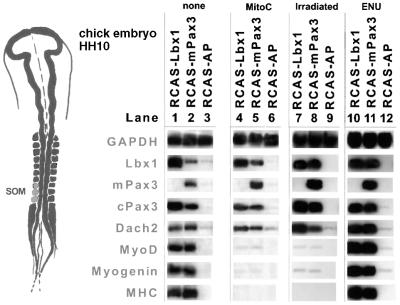
If the hypothesis is correct that a pivotal function of Lbx1 and Pax3 in the initiation of myogenesis is the increase in the pool of muscle precursor cells by stimulation of cell division, inhibition of cell proliferation should result in a repression of myogenesis. To test this postulate, somites I–IV from HH10 embryos were treated with mitomycin C for 6 h, 2 days after infection with RCAS(A)–Lbx1, RCAS(A)–Pax3 or RCAS(A)–hAP.
Four days later, tissue explants were fixed and stained with an antibody against PCNA to confirm the lack of dividing cells. We next monitored changes in the expression of myogenic markers and the Pax3–Lbx1–Dach2 regulatory loop by RT–PCR and immunohistochemical staining (Figure 6 and data not shown). Apparently, inhibition of cell proliferation resulted in a virtually complete lack of activation of the myogenic markers MyoD, myogenin and myosin heavy chain (Figure 6, lanes 4–6) despite a strong retrovirus-mediated expression of Lbx1 and Pax3. In contrast, the mutual stimulation of Lbx1 and Pax3 expression and the up-regulation of Dach2 mRNA occurred as in the control experiments in the absence of mitomycin C (Figure 6, lanes 1–3). In some experiments, a very low activation of myogenic differentiation markers was observed, correlating with the onset of mitomycin C treatment (Figure 6, lanes 4–6 and data not shown). This might be due to the fact that it was necessary to perform mitomycin C treatment 1–2 days after infection since efficient expression by RCAS-based retroviruses requires cell proliferation. To further confirm that Lbx1- and Pax3-induced cell proliferation is critical for activation of myogenic differentiation, tissue culture explants were irradiated with 50 Gy (5000 rads) 2 days after viral infection. This dose was sufficient to abrogate any cell divisions in explant cultures since no bromodeoxyuridine (BrdU)-incorporating or PCNA-positive cell was detectable after irradiation (data not shown). Similar to mitomycin C treatment, irradiation prevented the activation of myogenic markers by Lbx1 and Pax3 (Figure 6, lanes 7 and 8), but did not prohibit the mutual up-regulation of Lbx1 and Pax3 and the stimulation of Dach2 expression. It is feasible that treatment of explants with high doses of mitomycin C and radiation beyond the block of cell proliferation compromise cellular responses to an extent that activation of myogenic markers cannot occur anymore. Although this appears unlikely since the mutual up-regulation of Lbx1 and Pax3 and the stimulation of Dach2 expression occurred readily in treated and untreated cultures, we incubated explants with 0.25 mg/ml N-ethyl-N-nitrosourea (ENU) for 2 h. ENU is a potent mutagen, which has been demonstrated to generate high mutation rates in various types of cells (Chen et al., 2000). As shown in Figure 6, lanes 10 and 11, activation of myogenic markers in response to Lbx1 and Pax3 and in the presence of ENU occurred similarly to experiments without the drug, thus excluding the possibility that any cellular stress would block activation of myogenic markers in this type of experiment.
Fig. 6. Cell proliferation is required for Lbx1- and Pax3-mediated activation of myogenesis but not for Dach2 activation. Somites were dissected from HH10 embryos, separated from surrounding tissues and cultured in collagen gels. Tissues were infected with RCAS(A)–Lbx1 (lanes 1, 4, 7 and 10), RCAS(A)–Pax3 (lanes 2, 5, 8 and 11) and RCAS(A)–hAP (lanes 3, 6, 9 and 12) and cultured for 2 days. One set of tissues was left without additives (lanes 1–3), the other set was treated with mitomycin C (lanes 4–6) or irradiated (lanes 7–9) to inhibit cell proliferation. Another set of tissues was incubated with ENU (lanes 10–12). Four days after treatment, tissue explants were analyzed by RT–PCR. In cultures treated with mitomycin C or subjected to irradiation, expression of Lbx1 (lanes 4 and 7) and Pax3 (lanes 5 and 8) did not result in an up-regulation of MyoD, myogenin and MyHC, while treatment with ENU did not affect activation of myogenic markers (lanes 10 and 11).
The requirement of cell proliferation for Lbx1- and Pax3-induced myogenesis cannot be overcome by tissues inducing myogenesis
We reasoned that the failure of somitic explants to initiate myogenesis in the absence of further cell proliferation might be overcome by signals from neighboring tissues normally inducing myogenesis. Although it has been shown previously that a main input from adjacent tissues is the activation and/or maintenance of Pax3 expression (Maroto et al., 1997), it could not be excluded that the activation of other pathways contributes to the activation of myogenesis.
In order to include possible effects of signals from NT and the notochord in our analysis, we explanted somites I–IV from HH10 embryos together with these tissues and treated the explants with mitomycin C as before. As shown in Figure 7, myogenesis was not rescued in the presence of notochord and NT. None of the myogenic markers MyoD, myogenin and myosin heavy chain (Figure 7, lanes 3 and 4) was induced by RCAS(A)–Lbx1 and RCAS(A)–Pax3 despite a considerable up-regulation of Lbx1 and Pax3. Owing to the expression of Pax3, Lbx1 and Dach2 in NT, the basal levels of these markers were higher in the explants. It appears likely that this fact obscured the detection of a possible up-regulation of Dach2 in somites.
Fig. 7. The requirement of cell proliferation for Lbx1–Pax3-induced myogenesis cannot be overcome by tissues inducing myogenesis. Somites were dissected from HH10 embryos together with a segment of the adjacent NT and the notochord, and cultured in collagen gels. Tissues were infected with RCAS(A)–Lbx1 (lanes 1 and 3) and RCAS(A)–Pax3 (lanes 2 and 4) and cultured for 2 days. One set of tissues was left without additives (lanes 1 and 2), while the other set was treated with mitomycin C to inhibit cell proliferation (lanes 3 and 4). Four days after treatment, tissue explants were analyzed by RT–PCR. Similar to the experiment without muscle-inducing tissues, expression of Lbx1 (lane 3) and Pax3 (lane 4) did not result in an up-regulation of MyoD, myogenin and MyHC. To ensure that treatment of the NT–NC with mitomycin C or irradiation did not affect its ability to induce myogenesis in the PSM, the PSM was either cultured alone (lane 5) or with NT–NC that was not treated (lane 6), treated with mitomycin C (lane 7) or subjected to irradiation (lane 8). Myogenic marker molecules were induced in the PSM by the NT–NC (lanes 6–8), irrespective of a cell proliferation block in the NT–NC.
To rule out the possibility that the treatment of NT with mitomycin C affected its ability to induce myogenesis in the paraxial mesoderm, a number of control experiments were performed. PSM was dissected from HH10 chicken embryos and cultured alone or in combination with the NT–notochord complex (NT–NC). The tissues were harvested after 3 days and subjected to RT–PCR analysis. As described previously, myogenic marker molecules were induced only in the presence of NT–NC (Figure 7, lanes 5 and 6; Münsterberg et al., 1995; Maroto et al., 1997). When the PSM was combined with NT–NC that had been previously treated with mitomycin C or irradiated at 50 Gy (5000 rads), essentially the same results were obtained (Figure 7, lanes 7 and 8), demonstrating that cell cycle arrest did not affect the power of NT–NC to induce myogenesis in the PSM.
In conclusion, our experiments demonstrate that activation of the Pax3–Lbx1–Dach2 regulatory loop is not sufficient to initiate myogenesis, but requires an enlargement of the cell pool fated to become muscle. The requirement for this enlargement cannot be substituted for by signals from other tissues or by a combination of the Pax3–Lbx1–Dach2 regulatory loop and signals released by the notochord and NT.
Discussion
In this work, we used a gain-of-function approach in chicken embryos to assess the role of the homeobox transcription factor Lbx1 in initiation of myogenesis.
Lbx1 genetically acts upstream of myogenic factors and activates myogenesis in a cell type-dependent manner
The ability of muscle precursor cells to respond to environmental cues and to express mitogenic regulatory factors (MRFs) depends on numerous preceding decisions and the condition of a cell. Pax3 has been demonstrated to positively influence the capability of mesodermal cells for myogenic differentiation, although its mode of action is enigmatic (Maroto et al., 1997; Tajbakhsh et al., 1997). In this study, we have shown that Lbx1 has the same or even better potential than Pax3 to activate myogenesis. From our data, we conclude that Lbx1 is an important factor that might prepare and program muscle precursor cells to initiate myogenesis. In this regard, Lbx1 might substitute for Pax3 on a number of occasions. The ability of Lbx1 to induce myogenesis is cell type dependent, another property shared by Pax3. Expression of Lbx1 in 10T1/2 fibroblasts failed to induce myogenesis, while Lbx1 activated the myogenic program in primary chicken dermal fibroblasts as well as in NT explants and tissue fragments derived from the mesoderm. In vivo, the capacity of Lbx1 to activate the myogenic program is restricted, which is an interesting difference to the widespread activation of myogenesis in vitro.
Since forced expression of Lbx1 always induced Pax3, and vice versa, it cannot be excluded that both genes are necessary for the activation of myogenesis or that their respective targets mediate the effect of Lbx1 or Pax3. However, several arguments make the latter assumption more likely: (i) the activation of myogenic markers occurs more or less at the same time as the activation of Pax3 or Lbx1; (ii) in Lbx1 and in Pax3 mutant mice, the ability of muscle precursor cells to respond to Pax3 and Lbx1 is not lost; (iii) Lbx1 is expressed in Pax3-negative Mox2 mutant limb muscle precursor cells and apparently fulfills its role in hypaxial muscle formation independent of Pax3; and (iv) Lbx1 but not Pax3 can stimulate myogenesis in somites of intact embryos. Therefore, it appears more likely that both genes funnel into a common pathway, which seems to include Dach2, Eya2 and Six1 (Heanue et al., 1999).
It is tempting to speculate that primary chicken cells contain co-factors not present in 10T1/2 cells that are necessary for Lbx1-dependent activation of myogenesis but dispensable for the myogenic conversion of 10T1/2 cells by MRFs. It will be interesting to analyze whether the Dach2–Eya2–Six1 pathway, which might be instrumental for the effects of Lbx1 and Pax3, also operates in 10T1/2 cells.
Genetically, Pax3 is upstream of Lbx1 since Pax3 is expressed well before Lbx1 expression starts and the expression of Lbx1 is lost in somites of Pax3 mutant mice (Mennerich et al., 1998). In addition, in Lbx1 mutant mice, Pax3 can still be found in the remaining limb muscle precursor cell population. At first glance, it is therefore surprising that Lbx1 induces Pax3 even in the absence of cell proliferation. However, as outlined above, Pax3 seems to be necessary only for the initiation of Lbx1 expression but not for its maintenance. Lbx1, on the other hand, might be one of the factors by which Pax3 maintains its own expression via a positive regulatory feedback loop.
The regulation of cell proliferation by Lbx1 and Pax3 might be a key step in the control of myogenesis
The mechanism by which Lbx1 and Pax3 induce myogenesis in some cell types is still poorly understood. Considering the role of Pax3, different models have been discussed that are based on either the absence or presence of positive or negative regulatory co-factors, which may determine whether a particular tissue is prone to muscle cell formation or not. Lassar and colleagues suggested the existence of negative regulatory co-factors to explain the capability of Pax3 to activate myogenic factors in primary chicken cells but not in murine cells that are otherwise rather permissive for myogenesis (Maroto et al., 1997). The molecular control of cell proliferation has been largely neglected in this context, although it is well known that the control of the proliferation rate might decisively contribute to the control of myogenesis. The rate of cell proliferation decides whether a given cell population reaches a critical size that is capable of creating its own micro-environment, which, in turn, may support further proliferation and finally differentiation. In addition, continuous cell divisions may dilute negative co-regulators, thus enabling cells to differentiate. Under certain conditions, proliferation appears to be linked to a lasting suppression of differentiation, while other circumstances that promote cell division simultaneously keep, or probably even increase, the propensity of a cell to undergo myogenic specification. Lbx1 and Pax3 seem to fall in the second category.
We have shown that forced expression of Lbx1 and Pax3 stimulates proliferation of recipient cells, and that cell proliferation is a prerequisite for myogenesis, but not for the activation of the Pax3–Lbx1–Dach2 pathway. The time course of the appearance of proliferating cells in explants infected with RCAS–Lbx1 suggests that Lbx1 induces proliferation of quiescent cells. It cannot be ruled out, however, that Lbx1 maintains proliferation of cycling cells, but the initial decrease of proliferation followed by a sharp increase suggests that Lbx1 is able to recruit resting cells into mitosis.
We postulate that enhanced proliferation enlarges the muscle precursor cell pool and thereby augments its tendency for myogenic differentiation. Enlarging cell populations might become increasingly independent from repressing signals, such as BMP4, which originate from the ectoderm and keep hypaxial muscle precursor cells in an undifferentiated state (Pourquie et al., 1996; Amthor et al., 1998).
Our findings that Lbx1 and Pax3 stimulate cell proliferation in chicken embryo explants are in line with previous reports that murine Pax genes can promote oncogenesis in tissue culture cells and in mice (Maulbecker and Gruss, 1993). In addition, Sauvageau et al. (1995) have shown that overexpression of another homeobox-containing gene, HOXB4, in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. In a different approach, it has been demonstrated that loss of Pax3 is accompanied by a marked increase in programmed cell death (Borycki et al., 1999). Likewise, simultaneous inactivation of Pax3 and Pax7 leads to a strong reduction in the number of cells in somites and to virtually complete loss of myogenic differentiation in the myotome. Since apoptosis is a characteristic response of cells deprived of their appropriate stimuli, it is conceivable that the absence of the activation of cell proliferation leads to increased or even excessive programmed cell death, as in the case of Pax3 or Pax3–Pax7 double mutants. Hence, the lack of growth stimulation might be the cause for the increase in apoptosis.
A modified view on the community effect
The community effect has been described by Gurdon in Xenopus embryos (Gurdon, 1988). Single cells derived from a region of the gastrula fated to give rise to muscle and placed in an ectopic location fail to differentiate into muscle, whereas a group of cells will readily do so. Similarly, paraxial mesoderm cells isolated from the limb and somites of developing mouse embryos require a minimum number of cells to differentiate into muscle (Cossu et al., 1995). It has been speculated that the community effect might be based on autocrine or paracrine effects mediated by unstable or slowly diffusing substances or by cell–cell contacts. The concentration at which such molecules reach a certain threshold might be much higher in solid aggregates or clusters of cells than in single or dispersed cells.
Cell autonomous factors that act along this line must therefore meet two criteria: (i) able to stimulate cell proliferation to increase the size of the cell cluster; and (ii) able to support and maintain the myogenic potential of these cells. Lbx1 and Pax3 appear to fulfill both requirements, and might therefore induce myogenesis by creating a muscle precursor cell pool that is sufficient for initiation of the community effect.
This concept adapts previous findings that dissociated cells derived from the mesoderm or the chicken epiblast have an intrinsic tendency to form muscle (George-Weinstein et al., 1996). Dissociated mesodermal cells kept in culture will proliferate and form clusters of identical or related cells that differentiate spontaneously, following a similar fate as enforced by exogenous expression of Lbx1 and Pax3.
It is evident that such a mechanism is dependent on a strong intrinsic myogenic potential of mesoderm. Several observations, particularly in lower vertebrates, have suggested that myogenesis might be a default program of the mesoderm. For example, in Xenopus, MyoD transcripts are found in the oocyte and zygotic expression starts ubiquitously at mid-blastula transition (Harvey, 1990; Rupp and Weintraub, 1991). Later, during development, MyoD transcripts become restricted to muscle-forming regions, suggesting an onset of repression in prospective non-muscle cells and a lack of repression in future muscle cells. Finally, in the chordate amphioxus, the dermal segments, analogs of somites in vertebrates, lack the dermomyotome and the sclerotome, and completely differentiate into muscle. The dominance of the myogenic differentiation pathway is emphasized by the presence of sarcomeric proteins in mid-line cells of the notochord (Holland et al., 1995).
Refinement of the genetic hierachy that controls skeletal myogenesis
Numerous components that control myogenesis have been inactivated in the mouse by targeted mutation. How do our results fit into the genetic hierarchy defined by analysis of these loss-of-function mutants?
One of the principal defects of Lbx1 mutants is the failure of muscle precursor cells to migrate into their corresponding muscle anlagen (Schäfer and Braun, 1999). Expression analysis demonstrated that dislocated β-Gal+ cells initially expressed Pax3 and c-Met, although this ectopic expression faded quickly (Schäfer and Braun, 1999). No significant expression of the myogenic markers Myf5 and MyoD was found in ectopically located β-Gal+ cells, despite the ability of the remaining muscle precursor cells of forelimbs to switch on expression of Myf5 and MyoD. These findings are in line with a role of Lbx1 in the maintenance of Pax3 expression and the activation of myo genic bHLH genes in a subset of muscle precursor cells.
Myf5–Pax3 double mutants lack most of the muscles of the trunk and do not activate MyoD in the body. This dramatic phenotype is not seen in individual mutants. Therefore, it has been postulated that the expression of MyoD is dependent on both Myf5 and Pax3, and that these genes can mutually rescue each other (Tajbakhsh et al., 1997). Our results may provide an alternative explanation of how Pax3 might control myogenesis. We have shown that the stimulation of cell proliferation by Pax3, which leads to the amplification of myogenic progenitor cells, is required for the activation of MyoD and for myogenesis. In the absence of Pax3, Myf5 may exert a similar function, i.e. expand the population of muscle progenitor cells. In contrast to MyoD, which probably inhibits cell proliferation by activation of p21, Myf5 does not appear to restrict cell proliferation. MyoD–/– myoblasts continue to proliferate even in the presence of a 4-fold higher expression of Myf5 under conditions that normally induce differentiation (Sabourin et al., 1999). Hence, MyoD-expressing cells may be dependent on an additional factor that promotes cell proliferation. Pax3 (and in limb muscle, Lbx1) seems to be a good candidate for supplying such a function. In the absence of both Pax3 and Myf5, expansion of the myogenic lineage will not occur, resulting in a virtually complete absence of body muscles. This view is different from direct activation of the MyoD gene by Pax3 and focuses on the amplification of cells that subsequently express MyoD. Our interpretation is supported by results obtained in Pax3–Pax7 double-mutant mice, which show a strong reduction in the number of cells in the dermomyotome and the myotome, and a severe impairment of myogenic differentiation (A.Mansouri and P.Gruss, personal communication).
Materials and methods
Plasmids and retroviral vectors
Generation of viral constructs and production of high titer virus stocks were performed as described previously (Morgan and Fekete, 1996). Chick Lbx1 cDNA encoding the entire open reading frame was cloned by PCR, introducing an optimized translational initiation sequence and ClaI restriction sites. The retroviral construct encoding mouse Pax3 was a kind gift from A.Lassar (Maroto et al., 1997). RCAS(A)–hAP contained a cDNA of placental hAP. Retroviral titers ranged from 7 × 107 to 2 × 109 c.f.u./ml.
Virus injections and explant cultures
Injections of virus solutions in ovo were performed essentially as described by Morgan and Fekete (1996). Embryos were injected several times in separate somites, giving rise to distinct virus infection patterns. For explant cultures, embryonic mesoderm and neural tissues were isolated and cultured in collagen matrix as outlined by Münsterberg et al. (1995). Retroviral infections of explanted tissues were performed as described (Maroto et al., 1997). Cultures were incubated for 6 days and analysed by RT–PCR or immunohistochemistry.
In some experiments, explanted tissues were incubated for 6 h with 10 µg/ml mitomycin C 2 days after retroviral infection. Cultures treated this way were washed three times and incubated for another 4 days. For irradiation, explanted tissues were subjected to a dose of 50 Gy (5000 rads) from a cobalt source and cultured as above. Treatment with ENU was for 2 h in tissue culture medium supplemented with 0.25 mg/ml ENU, analogous to the mitomycin C incubation. Co-cultures of PSM with NT–NC were performed as described (Münsterberg et al., 1995) either with untreated NT–NC or with NT–NC that had previously been treated with mitomycin C or irradiated. To achieve BrdU incorporation, explants were incubated for several hours in 15 ng/ml BrDU in cell tissue culture medium 2 days after retroviral infection.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed according to the protocol of Wilkinson (1992), using appropriate cRNA probes. The retroviral Lbx1 transcripts were stained with an anti-fluorescein antibody coupled to peroxidase using Fast Red (Sigma) as a substrate. For simultaneous staining of MyoD or myogenin, the anti-fluorescein antibody was used together with an anti-digoxygenin antibody coupled to hAP and NBT–BCIP as a substrate, following the double-staining protocol developed by Hauptmann and Gerster (1994).
Analysis of cell proliferation
Proliferating cells in explanted tissues were determined by immunohistochemical stainings with antibodies against PCNA (Dakopads, Hamburg) or BrDU (Roche Biochemicals) as described (Krüger et al., 2001). To visualize bound antibodies, a biotinylated anti-mouse IgG secondary antibody was used with diaminobenzidine as substrate. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to reveal the absolute number of cells. To determine the number of proliferating cells, 48–72 viewing fields originating from 12 different explants were counted for each type of explanted tissue at each time point. Viewing fields were selected on a random basis. The total number of cells in each viewing field was usually between 90 and 120. The absolute number of proliferating cells per viewing field ranged between 2 and 95. Each viewing field was normalized for the total number of cells in the field. Induction of cellular proliferation is depicted as the ratio of proliferating cells between infected and uninfected control explants. The time course of proliferating cells in explanted tissues is shown as the ratio between proliferating cells and the total cell count.
RT–PCR
RT–PCR analysis was performed according to Krüger et al. (2001). Information about primers and conditions can be obtained from the authors.
Acknowledgments
Acknowledgements
We are indebted to S.Hoffmann and M.Schlegel for expert technical assistance, and to E.Bober and P.Neuhaus for critically reading the manuscript. We acknowledge the kind gift of the RCAS(A)–Pax3 retrovirus by A.B.Lassar and the full-length Pax3 cDNA by P.Gruss. This work was supported by grants to T.B. by the DFG.
References
- Amthor H., Christ,B., Weil,M. and Patel,K. (1998) The importance of timing differentiation during limb muscle development. Curr. Biol., 8, 642–652. [DOI] [PubMed] [Google Scholar]
- Arnold H.H. and Braun,T. (2000) Genetics of muscle determination and development. Curr. Top. Dev. Biol., 48, 129–164. [DOI] [PubMed] [Google Scholar]
- Bober E., Franz,T., Arnold,H.H., Gruss,P. and Tremblay,P. (1994) Pax3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development, 120, 603–612. [DOI] [PubMed] [Google Scholar]
- Borycki A.-G., Li,J., Jin,F., Emerson,C.P. and Epstein,J.A. (1999) Pax function in cell survival and in pax7 regulation. Development, 126, 1665–1674. [DOI] [PubMed] [Google Scholar]
- Chen Y., Yee,D., Dains,K., Chatterjee,A., Cavalconi,J., Schneider,E., Om,J., Woychik,R.P. and Magnuson,T. (2000) Genotype-based screen for ENU-induced mutations in mouse embryonic stem cells. Nature Genet., 24, 314–317. [DOI] [PubMed] [Google Scholar]
- Christ B., Jacob,H.J. and Jacob,M. (1977) Experimental analysis of the origin of the wing musculature in avian embryos. Anat. Embryol., 150, 171–186. [DOI] [PubMed] [Google Scholar]
- Cossu G., Kelly,R., Di Donna,S., Vivarelli,E. and Buckingham,M. (1995) Myoblast differentiation during mammalian somitogenesis is dependent upon a community effect. Proc. Natl Acad. Sci. USA, 92, 2254–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G., Tajbakhsh,S. and Buckingham,M. (1996) How is myogenesis initiated in the embryo? Trends Genet., 12, 218–223. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M. et al. (1996) Skeletal myogenesis: the preferred pathway of chick embryo epiblast cells in vitro. Dev. Biol., 173, 279–291. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B. (1988) A community effect in animal development. Nature, 336, 772–774. [DOI] [PubMed] [Google Scholar]
- Harvey R.P. (1990) The Xenopus MyoD gene: an unlocalised maternal mRNA predates lineage-restricted expression in the early embryo. Development, 108, 669–680. [DOI] [PubMed] [Google Scholar]
- Hauptmann G. and Gerster,T. (1994) Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet., 10, 266. [DOI] [PubMed] [Google Scholar]
- Heanue T.A., Reshef,R., Davis,R.J., Mardon,G., Oliver,G., Tomarev,S., Lassar,A.B. and Tabin,C.J. (1999) Synergistic regulation of vertebrate muscle development by Dach2, Eya2 and Six1, homologs of genes required for Drosophila eye formation. Genes Dev., 13, 3231–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L.Z., Pace,D.G., Blink,M.L., Kene,M. and Holland,N.D. (1995) Sequence and expression of amphioxus alkali myosin light chain (Amphi MLC-alk) throughout development: implications for vertebrate myogenesis. Dev. Biol., 171, 665–676. [DOI] [PubMed] [Google Scholar]
- Krüger M., Mennerich,D., Fees,S., Schäfer,R., Mundlos,S. and Braun,T. (2001) Sonic hedgehog is a survival factor for hypaxial muscles during mouse development. Development, 128, 743–752. [DOI] [PubMed] [Google Scholar]
- Maroto M., Reshef,R., Munsterberg,A.E., Koester,S., Goulding,M. and Lassar,A.B. (1997) Ectopic Pax3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell, 89, 139–148. [DOI] [PubMed] [Google Scholar]
- Maulbecker C.C. and Gruss,P. (1993) The oncogenic potential of Pax genes. EMBO J., 12, 2361–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerich D., Schäfer,K. and Braun,T. (1998) Pax3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech. Dev., 73, 147–158. [DOI] [PubMed] [Google Scholar]
- Morgan B.A. and Fekete,D.M. (1996) Manipulating gene expression with replication-competent retroviruses. Methods Cell Biol., 51, 185–218. [DOI] [PubMed] [Google Scholar]
- Münsterberg A.E., Kitajewski,J., Bumcrot,D.A., McMahon,A.P. and Lassar,A.B. (1995) Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev., 9, 2911–2922. [DOI] [PubMed] [Google Scholar]
- Ordahl C.P. and Le Douarin,N.M. (1992) Two myogenic lineages within the developing somite. Development, 114, 339–353. [DOI] [PubMed] [Google Scholar]
- Pourquie O. et al. (1996) Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell, 84, 461–471. [DOI] [PubMed] [Google Scholar]
- Rupp R.A. and Weintraub,H. (1991) Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X.laevis. Cell, 65, 927–937. [DOI] [PubMed] [Google Scholar]
- Sabourin L.A., Girgis-Gabardo,A., Seale,P., Asakura,A. and Rudnicki,M.A. (1999) Reduced differentiation potential of primary MyoD–/– myogenic cells derived from adult skeletal muscle. J. Cell Biol., 144, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau G., Thorsteinsdottir,U., Eaves,C.J., Lawrence,H.J., Largman,C., Lansdorp,P.M. and Humphries,R.K. (1995) Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev., 14, 1753–1765. [DOI] [PubMed] [Google Scholar]
- Schäfer K. and Braun,T. (1999) Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nature Genet., 23, 213–216. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Rocancourt,D., Cossu,G. and Buckingham,M. (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax3 and Myf-5 act upstream of MyoD. Cell, 89, 127–138. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Vivarelli,E., Cusella-De Angelis,G., Rocancourt,D., Buckingham,M. and Cossu,G. (1994) A population of myogenic cells derived from the mouse neural tube. Neuron, 13, 813–821. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.G. (1992) In Situ Hybridization: A Practical Approach. Oxford University Press, Oxford, UK.