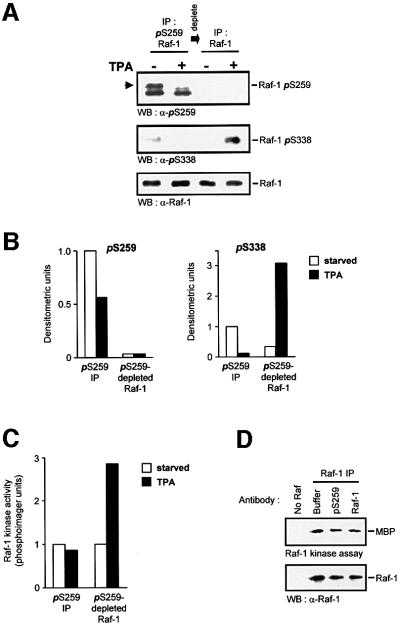
Fig. 2. Raf-1 phosphorylated on Ser259 is refractory to mitogen stimulation. Cells were lysed in RIPA buffer and Raf-1 was immunodepleted from serum-starved or TPA-treated (100 ng/ml for 20 min) cells using the affinity-purified anti-pSer259 antibody. The supernatant subsequently was immunoprecipiated with anti-Raf antiserum. Both fractions of Raf-1 were examined for phosphorylation of Ser259 and Ser338, using phosphospecific antisera (A and B), and for kinase activity (C). Raf-1 phosphorylated on Ser259 is indicated by the arrowhead; the lower of the two bands is non-specific. Similar results were obtained with EGF stimulation. For (B), the immunoblots of (A) were quantified by laser densitometry. The pSer259 and pSer338 signals, respectively, were normalized to the signals obtained with a non-discriminatory Raf-1 antibody (lowest panel in A). (D) The Raf-1 Ser259-phosphospecific antiserum does not inhibit kinase activity. COS cells were transfected with CMV5-Raf-1 and treated with 100 ng/ml TPA for 15 min. The cells were lysed in RIPA buffer and Raf-1 was immunoprecipitated with the c-Raf VI antiserum. Aliquots of the immunoprecipitate were incubated with either 2 µg of pSer259-specific or a non-discriminatory Raf-1 antibody (C-12; Santa Cruz) for 2 h. The kinase activities of the immunoprecipitates were then measured.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.