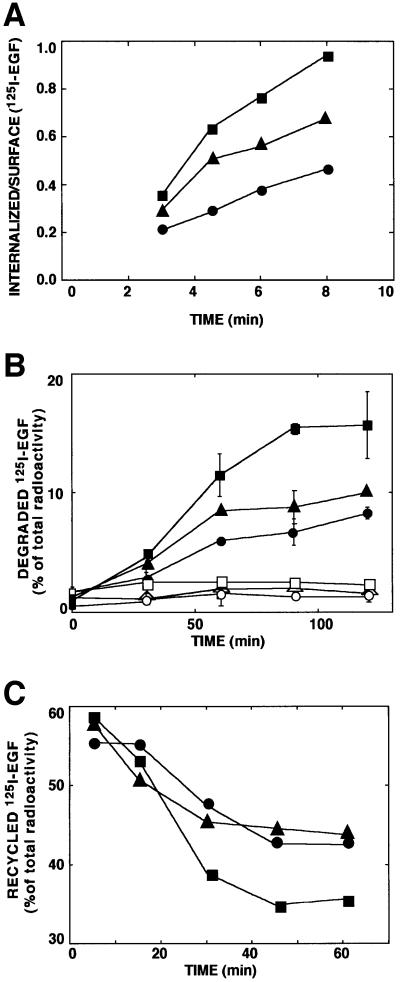
Fig. 3. The c-Cbl docking site of EGFR is essential for rapid ligand endocytosis and degradation, and it enables sequestration from the recycling pathway. (A) CHO cells transiently expressing a wt-EGFR (squares), a kinase-defective mutant receptor (circles) and a Tyr1045 mutant (Y1045F; triangles) were incubated at 37°C with a radiolabeled EGF (2 ng/ml). At the indicated times, cell monolayers were acid-washed to remove surface-bound EGF. Radioactivity present in the acidic fraction was quantified in triplicates and designated surface-associated ligand. The remaining cell-associated radioactivity (internalized) was similarly quantified following cell solubilization. The ratio obtained at each time point is presented (average ± SD). (B) Monolayers of CHO cells transfected as in (A) were incubated for 1 h at 20°C with a radiolabeled EGF. Sister monolayers were pre-incubated for 30 min with chloroquine (0.2 mM; open symbols). Thereafter, cells were washed and maintained at 37°C for the indicated time intervals. Media were then collected, and the cells were solubilized. The trichloroacetic acid-soluble radioactivity was determined and the fraction of degraded ligand presented. (C) CHO cells transiently expressing wt-EGFR (squares), the Y1045F mutant receptor (circles) or a kinase-defective mutant (triangles) were allowed to internalize a radiolabeled EGF (1 ng/ml) for the indicated time periods. The remaining surface bound ligand was removed by mild acid-washing. The EGF-loaded cells were then incubated with an unlabeled EGF at 4°C, followed by 1 h at 37°C. Intact radioactive ligand was harvested from the medium following a 1 h chase period. Similarly, the fraction of surface-bound radioactivity was determined and the combined fractions designated recycled ligand. The fraction of recycled ligand is presented as average ± SD (bars) of triplicate determinations.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.