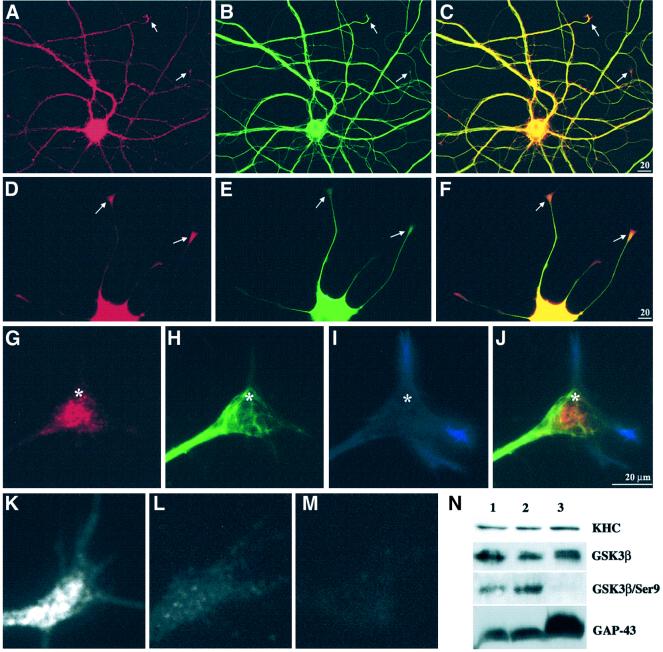
Fig. 7. GSK3 was increased at sites of active membrane delivery. A hippocampal neuron (A–C) and a differentiating PC12 cell (D–F) were double immunostained for GSK3β (A and D) and tubulin (B and E). In both cell types, the level of GSK3 was high in cell bodies and lower amounts were distributed along neurites. Merged images (C and F) highlight relative enrichment of GSK3β relative to tubulin at neurite tips (arrows). GSK3 immunoreactivity appears concentrated distal to MT ends. (G–J) High magnification of a triple-stained PC12 growth cone. Anti-GSK3β labels vesicle-like structures in the growth cone core (G), with a pattern distinct from MTs (H) and actin (I). In the merged image (J), the relationship of GSK3β to cytoskeletal elements is emphasized. Asterisks serve as reference points. Immunolocalization with an antibody that recognizes GSK3β independently of phosphorylation (active and inactive forms) shows enrichment of immunoreactivity in growth cones (K and G). In contrast, an antibody that only recognizes GSK3β phosphoSer9 (inactive kinase) (Wang et al., 1994a) is not enriched in growth cones (L). PhosphoSer9 immunoreactivity is abolished by phosphatase treatment (M), while it has no effect on staining with the phosphorylation-independent GSK3β antibody (K). Immunoblotting on proteins isolated from growth cones (N) confirmed the results of immunostaining. Lane 1 contains homogenate from E18 rat brain, lane 2 a 3000 g supernatant from E18 brain and lane 3 purified growth cone particles. The antibodies used on each of the four blots are indicated on the right.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.