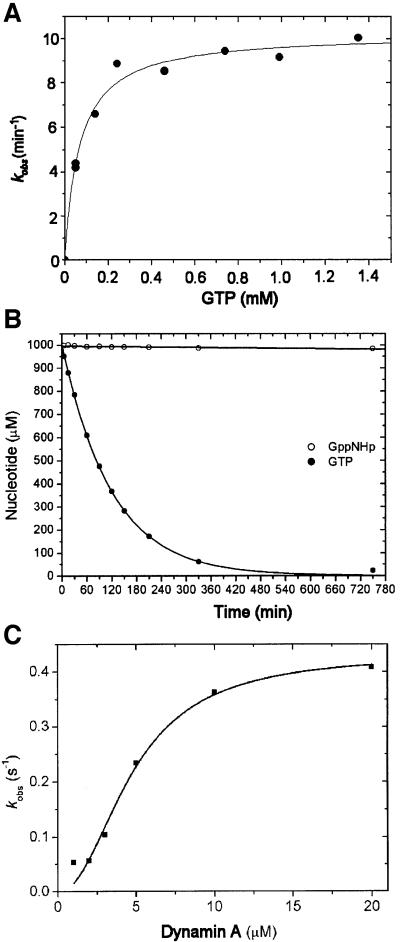
Fig. 1. GTPase activity of dynamin A. (A) GTPase activity of 1 µM dynamin A at 20°C as a function of GTP concentration. Values of 68 µM for KM and 0.17/s for kcat were determined with freshly purified dynamin A. (B) Hydrolysis of GTP and GppNHp by dynamin A*, the modified form of the enzyme. Complete hydrolysis of 1 mM GTP by 1 µM dynamin A* occurred with τ1/2 = 70 min at 20°C. No significant hydrolysis of 1 mM GppNHp could be detected following a 12 h incubation with 1 µM dynamin A at 20°C. (C) Cooperativity of the GTPase activity of dynamin A. The apparent kcat increased significantly with increasing dynamin A concentration. A fit of the data to the equation kobs = Vmax × [dynamin A]n/([dynamin A]n + kn) is shown, with Vmax = 0.43 ± 0.04/s, k = 4.75 ± 0.6 µM and n = 2.1 ± 0.4. The specific activity of dynamin A decreased upon storage at 80°C and up to 3-fold higher values for Vmax were measured with freshly purified protein.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.