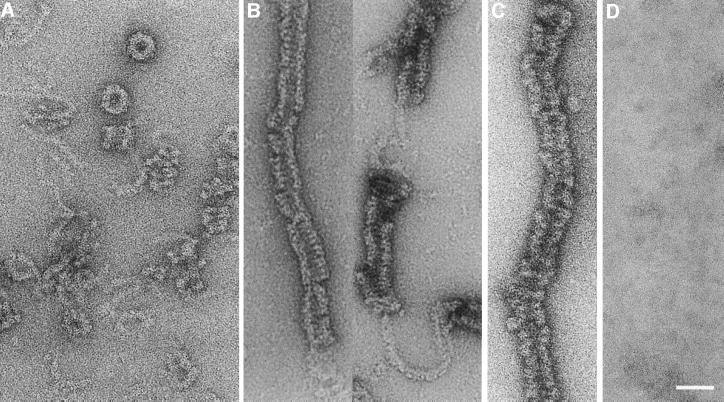
Fig. 3. Micrographs showing the nucleotide-dependent assembly behaviour of purified dynamin A. (A) In the absence of nucleotide, dynamin A forms round structures and loose filamentous structures that are frequently connected to the round structures or short helical assemblies. These structures are stable in 150 mM NaCl. (B) Addition of GppNHp induces the formation of long filamentous assemblies. The irregular nature of the assemblies makes it difficult to identify them unambiguously as either helices or stacks of closed rings; however, a helical nature is suggested by the frequent observation of ∼6 nm diameter density connecting adjacent regions of filamentous assemblies. (C) Long filamentous assemblies were also observed in the presence of GDP–AlFx. (D) Ring-like structures and other forms of assembled dynamin A were rarely observed in the presence of GDP, where unassembled protein appears to dominate. The scale bar represents 50 nm.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.