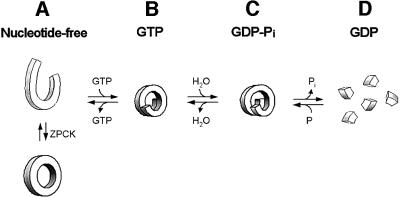
Fig. 8. Model of the mechanochemical cycle of dynamin A. The equilibrium between the different assembly states is highly nucleotide dependent. (A) In the absence of nucleotide, dynamin A forms strings of density ∼6 nm in diameter and round structures with a diameter of 32 nm. Modification by ZPCK stabilizes a uniform ring complex suitable for structural analysis. (B) Addition of GppNHp or GTP induces helix formation. (C) GTP hydrolysis is associated with a conformational change that leads to the disruption of contacts within the dynamin helix and ultimately to the dissociation into dimers in the GDP-bound state (D). The conformational changes upon GTP binding and GTP hydrolysis could cause constriction and stretching of the helix. Phosphate release appears to initiate helix disassembly. An increase in helical pitch has been predicted to occur, based on theoretical considerations (Kozlov, 1999) and was directly observed in the case of dynamin-1 (Stowell et al., 1999; Marks et al., 2001).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.