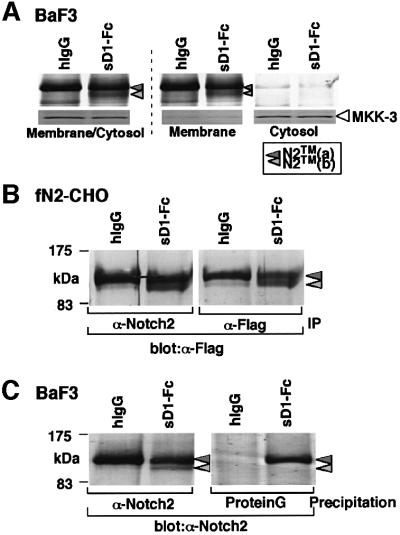
Fig. 3. Characterization of the N2TM(b) fragment induced by sD1–Fc-stimulation. (A) To determine whether N2TM(b) is a transmembrane protein, membrane/cytosol-rich fraction prepared from BaF3 after the sD1–Fc stimulation was then separated into membrane and cytosol fractions. N2 proteins in each fraction was subjected to western blot after immunoprecipitation with an anti-N2 polyclonal antibody. As a control for correct fractionation of membrane and cytosol franctions, an antibody against MKK3, MAP kinase, was used for each fraction in western blot analysis. (B) Generation of N2TM(b) fragment containing a Flag(His)6 tag at the C-terminus. fN2-CHO [CHO(r) with exogenous N2 with a Flag(His)6 tag at the C-terminus] was incubated in the presence of either sD1–Fc or hIgG at 6.7 nM. After 1.5 h, the stimulated cells were collected and solubilized in a TNE buffer. The cell lysates were precipitated with an anti-Flag monoclonal (M2) or an anti-N2 polyclonal antibody. The precipitates were analyzed by western blot with the M2 antibody. IP, immunoprecipitation. (C) Co-precipitation analysis. BaF3 was incubated in RPMI medium containing sD1–Fc or hIgG at 6.7 nM for 1.5 h, then subjected to a cross-linking reaction to form the binding complex of sD1–Fc and N2. Following the reaction, the BaF3 lysates were divided into two aliquots. One was precipitated with an anti-N2 polyclonal antibody to identify N2 protein fragments. To precipitate sD1–Fc-containing complex, protein G beads were added directly to the other. These precipitates were analyzed by western blot with the bhN6 antibody.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.