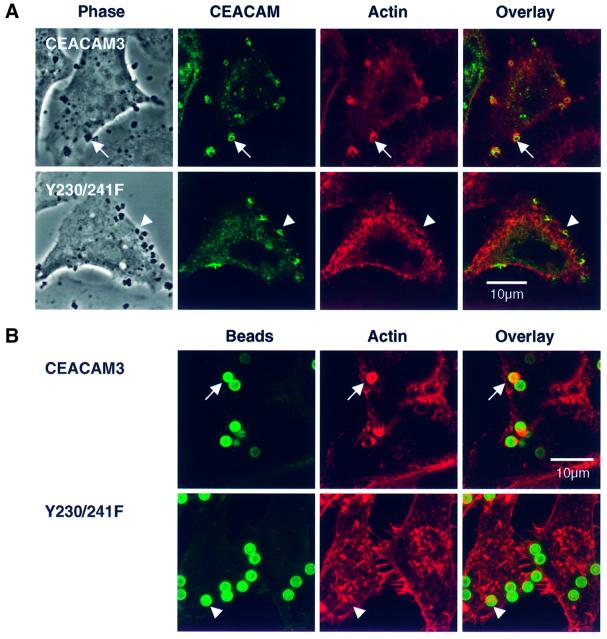
Fig. 6. The formation of phagocytic F-actin structures by CEACAM3 is triggered by receptor aggregation and critically depends on the tyrosine residues of the cytoplasmic receptor domain. (A) Confocal sections through COS-7 cells transiently transfected to express either CEACAM3 or the double mutant Y230/241F and infected with N313 (Opa57) for 20 min. Phase contrast shows cells and adhering bacteria. Immunofluorescence labelling of CEACAM receptors (green) reveals strong aggregation of both receptors at sites of bacterial adherence. Phagocytic F-actin structures as visualized by TRITC–phalloidin (red) are present in cells expressing wild-type CEACAM3 (arrows) but absent from cells expressing the Y230/241F double mutant (arrowheads). (B) Magnetic beads coated with the D14HD11 mouse monoclonal antibody against CEACAM receptors were incubated with HeLa cell lines for 20 min at 37°C at a ratio of five beads per cell. Receptor aggregation by anti-CEACAM beads is sufficient to induce phagocytic actin rearrangements in cells expressing CEACAM3 (arrows) but not in cells producing the Y230/241F double mutant. Beads were visualized in fixed and permeabilized samples by a dichlorotriazinylamino fluorescein (DTAF)-conjugated secondary antibody against the coating mouse monoclonal antibody.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.