Abstract
Hematologic malignancies most often present in the sixth or seventh decade of life. Even so, many older adults may be unable to tolerate standard chemotherapy or require supplementary care or dose adjustments to do so. Both in community and academic centers, geriatric assessment (GA) can be used to improve the care of older adults with blood cancers. For example, hematologic oncologists can use GA to guide treatment selection, adjusting for patient frailty and goals, as well as prompt initiation of enhanced supportive care. After initial therapy, GA can improve the identification of older adults with aggressive myeloid malignancies who would benefit from hematopoietic cell transplantation (HCT), inform shared decision making, as well as allow transplanters to tailor conditioning regimen, donor selection, graft-versus-host disease prophylaxis, and pre- and post-HCT treatments. As in HCT, GA can improve the care of older patients with relapsed lymphoma or multiple myeloma eligible for chimeric antigen receptor-T therapy, identifying patients at higher risk for toxicity and providing a baseline for subsequent neurocognitive testing. Here, we review the data supporting GA for the care of older adults with blood cancers, from the community to the academic center. In addition, we explore future directions to optimize outcomes for older adults with hematologic malignancies.
INTRODUCTION
Global life expectancy is projected to reach approximately 80 years by 2040. By 2030, adults age 65 years and older will account for 70% of all cancer diagnoses.1 Hematologic malignancies are the fourth most common cancer type, with 184,720 new cases projected in 2023.2 Leukemia, non-Hodgkin lymphoma (NHL), and multiple myeloma most commonly occur in older adults, with the median age of diagnosis ranging from 67–70 years.2 Even Hodgkin lymphoma, which is often a disease of younger adults, has a bimodal distribution such that 20.2% of diagnoses occur in adults age 65 years and older.2 Despite the increasing incidence of blood cancers with age, affected older adults represent a diverse population, with varying degrees of age-related concerns that can affect treatment decisions and outcomes. Furthermore, data for most novel therapies come from younger, healthier trial populations, and treatment guidelines are not tailored toward frail patients nor those with multimorbidity.3 The heterogeneity in the health status and functioning of older adults coupled with the growing armamentarium of treatment options demands an individualized approach to tailoring therapy in patients with hematologic malignancies.
In 1988, the National Institute of Health developed a consensus comprehensive geriatric assessment (cGA) to identify and assist in the treatment of older adults.4 Since this consensus cGA, parallel efforts have been made in oncology that retain all the core domains of a cGA, while increasing feasibility for busy oncology settings.5,6 In light of these recommendations, a thorough and collaborative assessment that (1) defines functional status, (2) outlines patient goals, and (3) identifies potentially modifiable weaknesses is needed to improve the care of the older adults with blood cancers.
CHALLENGES OF OLDER ADULTS WITH HEMATOLOGIC MALIGNANCIES IN THE COMMUNITY—IMPORTANCE OF EARLY REFERRAL
To appropriately treat older adults with hematologic malignancies, they must be assessed to determine their biologic age and fitness. This is best accomplished by using a screening GA for those age 65 years and older. Whether the Cancer and Aging Research Group (CARG) GA or the new ASCO practical geriatric assessment (PGA; Fig 1) is used depends upon time constraints and familiarity with each tool. Chronologic age alone and Karnofsky performance status, while useful, should supplement GA.7
FIG 1.

The PGA is a means to allow easier incorporation of GAs into everyday practice and has an estimated time to completion of 10–25 minutes. This assessment helps guide therapy decisions with the aim to provide better intradisciplinary communication, decrease treatment-related toxicities and hospitalizations, and improve patient/caregiver satisfaction. GA, geriatric assessment; PGA, practical geriatric assessment.
Although a GA may lengthen an office visit, the information gained is invaluable for treatment decision-making.8 The newly designed ASCO PGA has addressed time-constraint concerns and determined that incorporation of GA minimally affects office scheduling times.5,8 At baseline, 62% of patients age 65 years and older with NHL have dependence in one or more independent activities of daily living, 76.9% take at least four medications, and 47.5% have one or more comorbidities.7 GA-directed cancer therapy selection reduces treatment side effects, has been shown to be cost-effective, and can help predict survival.9–12
The treatment of adults with hematologic malignancies requires a robust infrastructure, and the management of older adults with these diseases also requires investment in resources. For instance, the treatment of leukemia requires a reliable blood bank, dedicated hematopathologists, oncology pharmacists, and technicians, an inpatient unit with specialized airflow, infectious disease specialists, pulmonologists, cardio-oncologists, intensivists, trained nurses, and a strong family or patient support network.13 To treat older adults, relationships with geriatric specialists is also necessary. However, the relative rarity of many hematologic malignancies prevents many community oncology centers from investing in infrastructure specific to these complicated cancer subtypes and subspecialties, including geriatric oncology.
Most centers that treat hematologic malignancies fall into one of three categories: academic medical centers, well-resourced community centers with dedicated hematologic malignancy programs, or under-resourced community centers or private offices. Given the lower incidence of hematologic malignancies and the rapidity with which novel therapies are being approved, an academic treatment center consultation may be helpful to weigh the risks and benefits of newer therapies, clinical trial participation, pursuit of chimeric antigen receptor (CAR)-T therapy, or consolidation with hematopoietic cell transplantation (HCT).
For newly diagnosed leukemia patients, academic medical center referral is recommended because of the high risk of mortality in the first few weeks to months after diagnosis and the need to initiate emergent and intensive care.14 In general, community centers should either seek input from academic clinicians or emergent referral to an academic center to treat acute leukemia. If not pursing intensive induction chemotherapy, azacitidine and venetoclax induction can be undertaken entirely outpatient at the community oncologist’s office if there are transfusion capabilities, but the input of a leukemia specialist is recommended throughout the treatment course. Indeed, the increasing use of outpatient therapies for leukemia necessitates that the community oncologist and the academic hematologic oncologist collaborate more closely than ever before. If not referred at diagnosis, all older patients with HCT-eligible myeloid malignancies should be referred to academic medical centers for evaluation immediately after induction.
Management of myelodysplastic syndrome (MDS) in the community is dependent upon appropriate pathologic and morphologic classification. Low-risk patients with cytopenias may require referral to academic medical centers if transfusion needs are unmet, therapy requirements are escalating, or progression to AML is suspected. All intermediate- or higher-risk MDS by Revised International Prognostic Scoring System should be referred to an academic center for consideration of allogeneic transplantation at diagnosis (with input from GA and patient values to inform this decision; see Section GA to Improve Treatment Decision Making in Older Patients With Hematologic Malignancies below). Similarly, patients with myeloproliferative neoplasms such as myelofibrosis (MF), polycythemia vera, and essential thrombocythemia may be treated in the community, but may require academic medical center consultation if response to initial therapy is inadequate or therapy selection is uncertain. All patients with newly diagnosed MF should have an academic medical center consultation for consideration of HCT, given the importance of timing HCT before the disease progresses to the point of affecting performance and organ function, which could worsen HCT outcomes or make patients HCT-ineligible.
In contrast to myeloid neoplasms, first-line treatment for multiple myeloma and lymphoma is often performed in the community. Once again, however, blood bank support and clinical expertise is critical, and there is a growing emphasis on assessing minimal residual disease (MRD) to determine appropriate treatment for these malignancies. The availability of high-quality imaging with positron emission tomography/computed tomography scan and dedicated radiographers is also essential.15 Autologous transplants for myeloma are often considered as part of initial care, and many relapsed patients with myeloma and lymphoma will require autologous HCT, bispecific T-cell engaging therapy (BiTE), and CAR-T therapy, for which an academic medical center referral is needed. Given the logistical challenges of CAR-T therapy, referral is recommended when a patient first relapses, rather than delaying until after multiple rounds of lymphodepleting chemotherapy that may hamper T-cell collection. A GA before referral is invaluable to assess baseline cognitive function and can be useful for long-term follow-up. When considering BiTE therapy, given similar logistical difficulties and adverse effects to CAR-T, patients should also be referred to an academic medical center.16,17
Although various barriers exist to the treatment of older adults with hematologic malignancies in the community, many can be overcome with a strong relationship with an academic medical center. With this close collaboration and the increasing use of GA to inform treatment selection, prompt initiation of supportive care, guide timing of referral, and clarify patient goals, community and academic teams can work together to truly personalize the care for the older adult with blood cancer.
GA TO IMPROVE TREATMENT DECISION MAKING IN OLDER PATIENTS WITH HEMATOLOGIC MALIGNANCIES
Multiple studies have proven the value of GA measures as predictors in older adults with blood cancers.18–22 However, to illustrate how GA can tailor treatment decisions, we first look to recent evidence. Two large randomized controlled trials (RCTs) that included older adults with predominantly solid cancers demonstrated that GA improves treatment decision making. The Geriatric Assessment for Patients 70 years and older (GAP70+) trial examined whether GA could reduce serious toxicity in older patients with advanced cancer receiving treatment.12 The Geriatric Assessment-driven Intervention (GAIN) trial investigated if GAIN could reduce grade 3 or higher chemotherapy toxicity in older adults with cancer.23 Both trials showed that GA integrated alongside standard oncologic care reduces severe cancer treatment toxicity without compromising treatment efficacy.12,23 Two mechanisms have been hypothesized by which GA mediates these outcomes (1) better matching of cancer treatment intensity (eg, treatment modality, and number and dosing of drugs) with patient function and (2) enhancing supportive care interventions aimed at reversing any components of frailty. Further analysis of GAP70+ showed that, compared with those receiving standard oncologic care, older patients receiving GA-guided care received, on average, more primary treatment modifications, which in turn were associated with a 15% reduced risk of severe toxicity and a 20% reduced risk of patient-reported functional decline.24 GAIN did not show that GA influenced the intensity of initial cancer treatment, but did show a substantial effect of GA in increasing the number and types of supportive care interventions aimed at minimizing the negative effects of intense treatments.23
Importantly, it is difficult to disentangle effects of supportive care from effects of treatment modifications for older adults with cancer. For example, more upfront supportive care interventions may improve frailty, boosting physiologic reserve and the ability to tolerate cancer treatment regimens at the standard doses studied in younger patients.25 However, upfront treatment modifications may result in less need for supportive care. What is clear from GAP701 and GAIN is that GA can help oncology teams tailor therapy to minimize both overtreatment of a patient’s cancer—similar cancer control with fewer adverse events—and undertreatment of aging-related health deficits, regardless of what cancer treatment is chosen.26
There were few older adults with blood cancers in the above trials (6% lymphoma in GAP70+, none reported in GAIN), and to date, to our knowledge, only one published RCT has focused on investigating geriatrics-guided care in a population of older adults with blood cancers.27 This study randomized prefrail and frail transplant-ineligible patients age 75 years and older with leukemia, lymphoma, and multiple myeloma to receive standard oncologic care plus consultation with a geriatrician versus standard oncologic care alone. The frailty designation was determined by a trained research assistant using a combined Fried/Rockwood model. The study did not demonstrate a benefit of geriatrics consultation on overall survival or unplanned hospitalizations; however, patients in the geriatrician consultation arm received multiple supportive care interventions addressing comorbidities, polypharmacy, function, falls, and cognition. Moreover, patients in the geriatrician arm were more likely to have end-of-life and goals-of-care discussions compared with patients in the standard arm. In addition, surveyed hematology-oncology clinicians whose patients participated in geriatric consultation found it useful in supporting management of frailty and, to a lesser degree, in informing cancer treatment decisions. Unlike GAP70+, which included explicit recommendations for oncologists to consider cancer treatment modifications in the presence of GA deficits, the hematologic malignancies RCT intervention allowed the treating oncologist to make treatment modifications on the basis of their applications of findings from the geriatric medicine consultation.
A few nonrandomized studies have reported mixed results regarding the effectiveness of GA in tailoring decision making among older adults with blood cancers. For example, Lin et al28 demonstrated that in a cohort of patients with relapsed/refractory large B-cell lymphoma undergoing CAR-T therapy (n = 75), those who received geriatric consultation experienced no improvement in length of inpatient stay or rate of unplanned care utilization compared with those who did not. However, receipt of geriatric medicine consultation was associated with a lower risk of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, hypothesized to be mediated by better identification and management of GA deficits that also confer a higher risk of both. Derman et al29 reported that, compared with a historical cohort, older patients with blood cancers referred for HCT and adoptive T-cell therapy who underwent GA in a multidisciplinary clinic experienced fewer deaths in hospital, shorter hospital length of stay, and fewer discharges to nursing facilities. The clinic provided GA-guided recommendations targeting identified frailty deficits and provided one of three GA-tailored treatment recommendations: (1) optimize and proceed with HCT or adaptive T-cell therapy; (2) optimize and decline HCT since achieving acceptable resilience is unlikely; and (3) optimize and defer HCT until established improvements are met upon reevaluation in the clinic. The authors hypothesized that the improved outcomes observed for those who received GA-guided care before HCT were due to both improved optimization of frailty as well as better selection of older patients suitable to undergo HCT or cellular therapy.
The available evidence suggests that GA improves treatment decisions in older adults. Using MDS as an example, while bone marrow assessment is necessary to classify MDS risk, MDS risk classification alone is insufficient to inform treatment recommendations (Fig 2A). For example, in a 74-year-old with high-risk MDS, given that data on safety and harms of intensive therapy are mostly from younger patients, some oncologists might avoid referral for HCT on the basis of the patient’s age alone.30,31 Yet, not offering HCT may represent undertreatment (Fig 2B) if the patient is independent in ADLs and instrumental activities of daily living (IADLs), still actively volunteering in their community with a good social support system, has limited comorbidities, and prioritizes aggressive cancer control with the aim of living as long as possible.26 HCT has been shown to improve overall survival and leukemia-free survival in adults up to age 75 years when compared with conventional care.32 Conversely, aggressively treating to minimize risk of progression of anemia and conversion to AML recurrence may represent overtreatment if the same patient were cognitively impaired and frail (Fig 2C). Even hypomethylating agents may be overtreatment if they were asymptomatic from MDS, with consistently stated goals of remaining out of the hospital and minimizing trips to the clinic to maximize time at home with family (Fig 2D). Indeed, determining if and when to start therapy, which agent to use and at what dose, whether the patient has comorbidities that preclude the use of certain drugs, and whether they have the function and social support necessary to adhere to a prescribed treatment plan, all depend just as much on a rigorous assessment of the patient’s health status as on morphologic and cytogenetic assessment of their marrow.33
FIG 2.
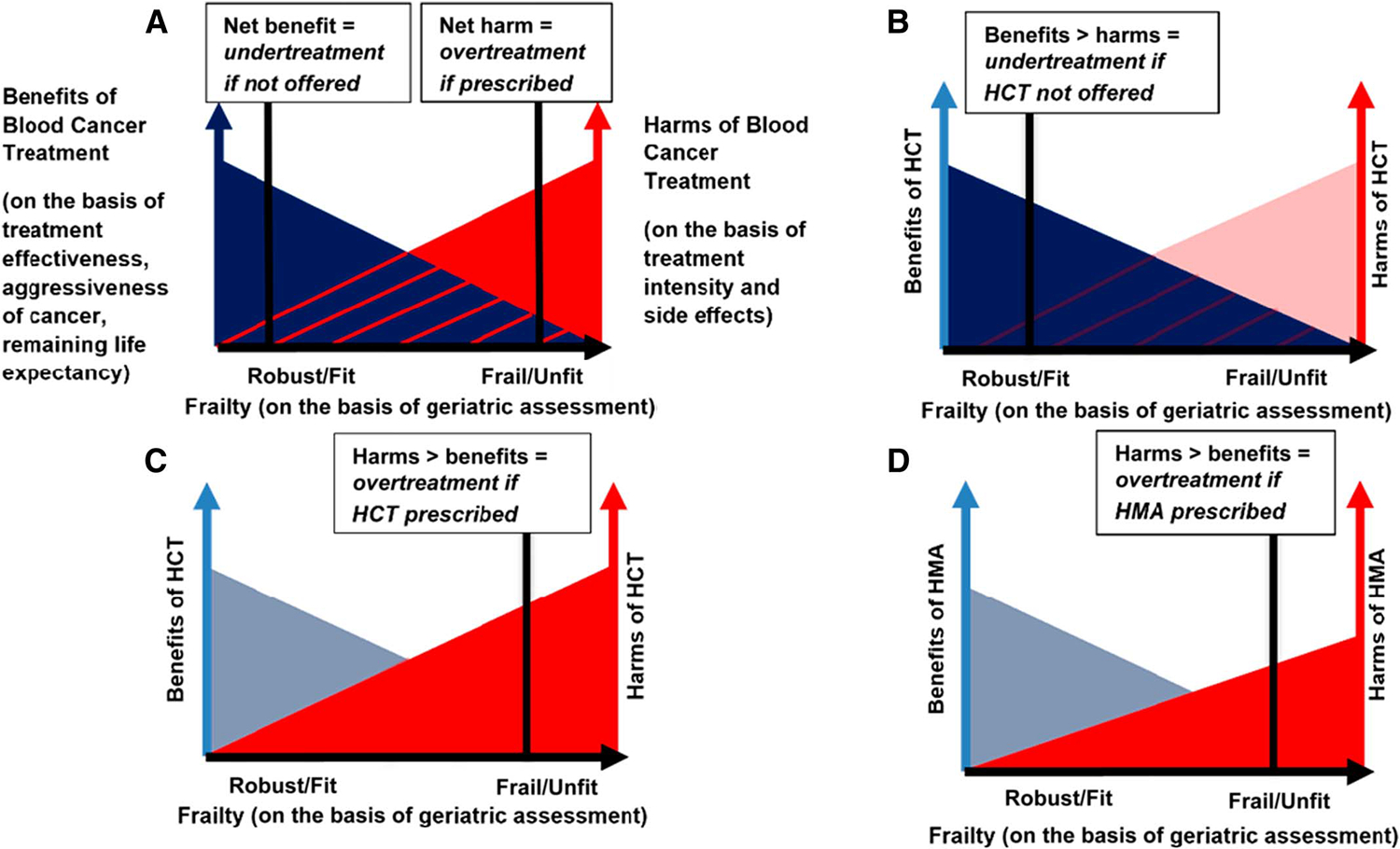
Graphical illustrations showing how assessment of frailty, on the basis of GA, is necessary to determine whether a given blood cancer treatment is optimally tailored to an older adult. (A) General graph of relationships among benefits of blood cancer treatment, harms of blood cancer treatment, and frailty of older patient. Benefits of blood cancer treatment are a function of the effectiveness of the treatment, the aggressiveness of the blood cancer, and the remaining life expectancy of the patient. Harms of a particular treatment are a function of treatment intensity and adverse effects. Frailty is a function of GA deficits (eg, cognitive impairment and functional dependency). As patient frailty increases, blood cancer treatment benefits generally decrease and harms increase. For a given level of frailty on the x-axis, the dark gray shading represents a treatment where the benefits outweigh the harms (net benefit = undertreatment if not offered). For more severe frailty on the x-axis, the red shading represents a treatment where the harms outweigh the benefits (net harm = overtreatment if prescribed). Individual patient preferences should inform the balance between benefits and harms of a given treatment. (B) A 74-year-old man with high-risk MDS, who is fit (functionally independent, cognitively intact, and few comorbidities) and values aggressive cancer control and prolongation of survival. Not offering HCT on the basis of age alone would be undertreatment in this patient. (C) A 74-year-old man with high-risk MDS, who is frail (functionally dependent in all instrumental activities of daily living because of advancing dementia). HCT would be overtreatment in this patient. (D) A 75-year-old man with high-risk MDS, who is frail (cognitively intact, but functionally dependent in some instrumental activities of daily living because of advanced heart failure) and values minimizing additional treatments and maximizing time at home. Prescribing a hypomethylating agent may confer lower risk of side effects than HCT, but may still be overtreatment, given the patient’s frailty and values. GA, geriatric assessment; HCT, hematopoeitic cell transplantation; HMA, hypomethylating agent; MDS, myelodysplastic syndrome.
There are multiple different forms of GA-guided care, including a multidisciplinary clinic implementing GA findings, staff completing screening GA and providing recommendations to oncologists, and embedding a geriatrician within the oncology team. As long as core elements of the GA, such as those supported by ASCO, are administered, the GA care model can be adapted to community clinic resources and staff.34 GA can only be effective at aiding oncology teams to tailor treatment decisions if performed early, upstream in decision making, and with high fidelity across patients. Trials investigating GA that have observed mixed effects on outcomes (such as the RCT in blood cancers discussed above) have cited delays in administering the GA and/or in communicating its findings as limitations that hindered its effectiveness.27,35 Another barrier to implementation of GA is that many oncologists cite a lack of knowledge on how to interpret its many findings, making it difficult to account for them in treatment decisions.36,37 More data are needed to assess the impact of cognitive decline, functional limitations, and frailty on cancer outcomes as well as the effects of modifying cancer treatment on the basis of identified impairments.
Promisingly, more trials are enrolling not only older adults, but also older adults with frailty and multimorbidity.36 There is also increasing emphasis on including measures and end points meaningful to older adults in clinical trials, such as patient-reported outcomes and quality of life.38–40 Improvements in progression-free survival or response rates may be irrelevant to certain older adults if these surrogate end points do not translate into functional or quality-of-life benefits.41,42 Trials randomly assigning older adults to varying treatment intensities on the basis of frailty, such as the UK fitness trial,43 will provide direct evidence demonstrating whether less-intense treatment options provide similar benefits with fewer harms. Well-designed observational studies will also be needed to fill the gaps for the frailest patients unlikely to participate in trials. For example, recent evidence suggested that more intensive treatment of multiple myeloma (eg, with the triplet bortezomib, lenalidomide and dexamethasone) may be of greater net benefit to patients with high levels of frailty compared with less-intensive treatment (eg, lenalidomide and dexamethasone).44 Finally, treatment strategies tested in older patients with blood cancers with specific health deficits, such as functional dependency, cognitive impairment, and mobility limitations, will be needed to address safety concerns for these high-risk patients. However, without increasing the use of GA measures in research and clinical practice, we will not be able to personalize and improve the treatment of older adults with blood cancers.
OPTIMIZING ALLOGENEIC TRANSPLANTATION AND EXTENDING THE AGE BARRIER TO TRANSPLANT
HCT represents the only curative option for many patients with blood cancers. Improvements in conditioning, supportive care, and graft-versus-host disease (GVHD) prevention have led to a decline in transplant-associated mortality (TRM) from 30% in 1980 to 10% in 2020.45 Given its increased safety, HCT is considered standard of care for most patients age 75 years or younger, and several centers have no age limit for HCT consideration.46
Reduced-intensity conditioning (RIC), by decreasing TRM, paved the way to HCT for many adults age 60 years and older. In contrast to myeloablative conditioning (MAC), which uses high-dose chemotherapy that carries significant risk of organ damage and death, RIC uses myelosuppressive doses that enable engraftment with less TRM and relies more heavily on graft-versus-tumor effects for relapse prevention. In older adults with AML in complete remission without MRD after intensive induction chemotherapy, RIC HCT has been associated with improved overall survival relative to conventional consolidation and equivalent survival to MAC HCT.47 RIC HCT also represents a viable treatment option for older adults with chronic myeloid malignancies. For example, in patients with higher-risk MDS age 60–75 years, HCT was associated with a 21% improvement in 3-year overall survival when compared with conventional care.32
Just as conditioning for HCT is getting less intense, so too are induction treatment options for AML. For instance, the intermediate-intensity combination of azacitidine and venetoclax leads to complete remission for 67% of older adults with AML, many of whom are unfit for intensive induction.48 These older adults with AML who achieve complete remission after nonintensive strategies may then be considered for consolidative RIC HCT. Although MRD after intensive induction chemotherapy worsens post-HCT survival, particularly when using RIC rather than MAC, MRD after azacitidine and venetoclax does not appear to negatively affect survival after RIC HCT.49 Less intensive and/or targeted induction strategies may also have the benefit of preventing functional declines associated with conventional chemotherapy, while still achieving effective disease control to allow for potentially curative RIC HCT. This will hopefully allow the broader implementation of RIC HCT as well as lead to reductions in TRM and improved OS for older adults, or indeed adults of any age with comorbidities, undergoing HCT.50
In a further boon to the HCT field, ruxolitinib, a Janus kinase 1/2 (JAK) inhibitor, when taken in the peritransplant setting until engraftment, decreases TRM and acute GVHD, improving overall survival for patients with high-risk MF.51,52 Ruxolitinib also represents an effective treatment option for steroid-refractory acute and chronic GVHD, thereby improving the safety of HCT by increasing effective treatment options for its potential complications. Moreover, its success in the treatment of GVHD and in the periengraftment setting for MF has paved the way for new studies incorporating ruxolitinib into GVHD prophylaxis, with promising early results.53
Another dramatic shift in HCT in recent years has come with the widespread adoption of high-dose post-transplant cyclophosphamide (PTCy)–based GVHD prophylaxis. PTCy-based GVHD prophylaxis is associated with improved GVHD-free, relapse-free survival when compared with tacrolimus and methotrexate-based GVHD prophylaxis, at 52.7% versus 34.9%, respectively, in a RCT of RIC HCT.54 Of note, in this trial, 56.8% of patients were age 65 years or older.54 Although it effectively reduces severe acute and chronic GVHD, high-dose PTCy is not without side effects. PTCy has been associated with delayed engraftment, higher rates of cardiac events, and greater infectious risk.55–57 Given the susceptibility of older adults to those complications, Duléry et al58 investigated the use of reduced-dose PTCy (80 mg/kg compared with standard 100 mg/kg) in patients age 65 years and older undergoing haploidentical HCT and demonstrated improved engraftment and survival when compared with historical controls. The use of intermediate-dose PTCy in older or frail HCT recipients is being explored in ongoing trials, again with promising early results (ClinicalTrials.gov identifier: NCT04959175).59
Agents such as PTCy and ruxolitinib, which may prevent GVHD, are particularly important for older adults who have a higher incidence of GVHD and are more vulnerable to morbidity and mortality from GVHD and its treatments. Abatacept, a cytotoxic T-cell lymphocyte-4 immunoglobulin, is another avenue to reduce acute GVHD that has been approved for HLA-mismatched unrelated donor HCT and may improve the safety of HCT for older adults.60 Future trials will likely combine JAK inhibitors, PTCy, abatacept, and novel agents such as belumosudil to further decrease GVHD and TRM after HCT and increase its applicability to older or frail recipients.
While increasing the safety of HCT will allow us to extend the age barrier to HCT, use of GA will enable us to personalize HCT for older adults.61 The most widely used metric to risk stratify HCT candidates is the HCT-specific comorbidity index (HCT-CI), which uses comorbidities and organ function to predict risk. Although helpful, the HCT-CI is most accurate in patients with a score of 2 or less, and overestimates NRM in those with an HCT-CI of ≥3.62 Of note, the median age in the validating study was 47 years.62 In a recently accrued multi-institutional Blood and Marrow Transplantation Clinical Trials Network study (BMT CTN 1704, Composite Health Assessment Risk Model or CHARM), additional factors such as weight loss over the past year, patient-reported Karnofsky performance status, PROMIS physical function scale, IADL, number of falls, PROMIS depression scale, number of prescribed medications, 4-m walk speed, cognition by the Montreal Cognitive Assessment (MoCA), C-reactive protein, and albumin were added to HCT-CI in an effort to improve risk stratification of older adult HCT recipients.61
Outside of comorbidity assessment, physical function identified as part of GA has been the best predictor of survival and toxicity in HCT patients.63 One measure of function and vulnerability is frailty. Frailty is a state of multiorgan physiologic dysregulation and decreased functional reserve that leaves patients susceptible to disability and death.64 Age and frailty are not linearly related, and studies have shown that frailty predicts death more accurately than chronologic age.65 Fried’s frailty phenotype (FFP) is a tool to identify frailty that uses five components: weight loss, weakness, exhaustion, slowness, and low physical activity level.66 Patients with scores of 0 are fit, 1–2 are prefrail, and 3+ are frail. Sung et al67 evaluated FFP as a tool to predict overall survival in older HCT patients. They found that FFP score was significantly associated with overall survival in older adults and could be combined with age, Karnofsky performance, and disease risk index to create distinct risk groups.67 Importantly, frailty is a potential target for intervention, and ongoing trials are exploring prehabilitation as well as rehabilitation in older adults undergoing HCT (ClinicalTrials.gov identifier: NCT05612789). Although for many centers an age limit for HCT still exists, with new treatments at our disposal, a well-rounded approach to a GA, and promising ongoing trials, more older adults will be able to undergo HCT safely than ever before.
SUMMARY
New targeted medications, less-intensive induction options, novel HCT platforms, and the growing understanding of how to leverage GA to optimize patient selection have improved outcomes for older adults with blood cancers. Advances in chemotherapeutics and cellular therapies have also led to increased safety and efficacy, allowing a wider spectrum of patients with blood cancers to undergo disease-directed therapies. For example, azacitidine and venetoclax, by inducing remissions in older patients with adverse cytogenetic or molecular risk who are unfit for intensive induction chemotherapy, are increasing the number of patients with AML achieving CR and thus becoming eligible for consolidative HCT.50 In HCT, innovative platforms are increasing the safety of HLA-matched and HLA-mismatched HCT, expanding this potentially curative treatment to an older and less fit population. These novel approaches accompanied with GA-guided identification of patient vulnerabilities and increased emphasis on intervening to optimize patient function are breaking down age barriers and improving outcomes for older adults with hematologic malignancies.
CONCLUSION
Although we need trials that will generate data specific to frail older patients with hematologic malignancies, current evidence supports the use of GA when determining treatment paradigms in older adults. In fact, GA is an important component in all levels of care for older patients with blood cancers. In the community, GA can be used to select treatment intensity, inform the decision to refer to an academic medical center, or to intervene on identified geriatric deficits to lessen toxicity. In academic medical centers, GA can help determine eligibility for induction or consolidative strategies, such as CAR-T or HCT, and guide optimization of patient’s performance, quality of life, and function before and after intensive treatments. As such, oncologists must incorporate GA into their practice patterns in order to inform and improve the care for older adults with blood cancers.
PRACTICAL APPLICATIONS.
In the community and at academic centers, geriatric assessment (GA) can guide shared decision making and improve outcomes in older adults with hematologic malignancies.
GA can inform treatment selection, supportive care implementation, and referral for multidisciplinary support in newly diagnosed patients.
GA can prompt optimization strategies or prehabilitation programs before hematopoietic cell transplantation (HCT) or chimeric antigen receptor (CAR) T-cell therapy.
With advances in the safety of HCT and CAR T and the growing use of GA to select appropriate candidates, we may eradicate the age barrier to these curative therapies.
Footnotes
Brendan L. Mangan
Honoraria: Pharmacy Times
Consulting or Advisory Role: Prime Meridian Group
Clark DuMontier
Stock and Other Ownership Interests: Lilly
Gregory A. Abel
Consulting or Advisory Role: Novartis
Shannon R. McCurdy
Consulting or Advisory Role: Bristol Myers Squibb Foundation
Research Funding: Gilead Sciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Foreman KJ, Marquez N, Dolgert A, et al. : Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392:2052–2090, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2020). National Cancer Institute. 2022. www.seer.cancer.gov/popdata [Google Scholar]
- 3.Sedrak MS, Freedman RA, Cohen HJ, et al. : Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin 71:78–92, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Conference Statement: Geriatric assessment methods for clinical decision-making. J Am Geriatr Soc 36:342–347, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Dale W, Klepin HD, Williams GR, et al. : Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline update. J Clin Oncol 41: 4293–4312, 2023 [DOI] [PubMed] [Google Scholar]
- 6.Outlaw D, Abdallah M, Gil-Jr LA, et al. : The evolution of geriatric oncology and geriatric assessment over the past decade. Semin Radiat Oncol 32:98–108, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhtar OS, Huang LW, Tsang M, et al. : Geriatric assessment in older adults with non-Hodgkin lymphoma: A Young International Society of Geriatric Oncology (YSIOG) review paper. J Geriatr Oncol 13:572–581, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams GR, Hopkins JO, Klepin HD, et al. : Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline questions and answers. JCO Oncol Pract 19:718–723, 2023 [DOI] [PubMed] [Google Scholar]
- 9.Scheepers ERM, Vondeling AM, Thielen N, et al. : Geriatric assessment in older patients with a hematologic malignancy: A systematic review. Haematologica 105:1484–1493, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishijima TF, Shimokawa M, Komoda M, et al. : Survival in older Japanese adults with advanced cancer before and after implementation of a geriatric oncology service. JCO Oncol Pract 19: 1125–1132, 2023 [DOI] [PubMed] [Google Scholar]
- 11.Sahakyan Y, Li Q, Alibhai SMH, et al. : Cost-utility analysis of geriatric assessment and management in older adults with cancer: Economic evaluation within 5C trial. J Clin Oncol 42:59–69, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohile SG, Mohamed MR, Xu H, et al. : Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP701): A cluster-randomised study. Lancet 398:1894–1904, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jillella AP, Cortes JE, Kota VK: Optimizing management of acute leukemia in community centers and when to refer. Hematol Am Soc Hematol Educ Program 2020:123–128, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantarjian H, Kadia T, DiNardo C, et al. : Acute myeloid leukemia: Current progress and future directions. Blood Cancer J 11:41, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoster E, Delfau-Larue MH, Macintyre E, et al. : Predictive value of minimal residual disease for efficacy of rituximab maintenance in mantle cell lymphoma: Results from the European Mantle Cell Lymphoma Elderly Trial. J Clin Oncol 42:538–549, 2024 [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Liu M, Ren F, et al. : The landscape of bispecific T cell engager in cancer treatment. Biomark Res 9:38, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann MS, Hunter BD, Cobb PW, et al. : Overcoming barriers to referral for chimeric antigen receptor T cell therapy in patients with relapsed/refractory diffuse large B cell lymphoma. Transplant Cell Ther 29:440–448, 2023 [DOI] [PubMed] [Google Scholar]
- 18.Min GJ, Cho BS, Park SS, et al. : Geriatric assessment predicts nonfatal toxicities and survival for intensively treated older adults with AML. Blood 139:1646–1658, 2022 [DOI] [PubMed] [Google Scholar]
- 19.Merli F, Luminari S, Tucci A, et al. : Simplified geriatric assessment in older patients with diffuse large B-cell lymphoma: The prospective Elderly Project of the Fondazione Italiana Linfomi. J Clin Oncol 39:1214–1222, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Klepin HD: Toward consensus on geriatric assessment in AML. Blood 139:1605–1606, 2022 [DOI] [PubMed] [Google Scholar]
- 21.Cordoba R, Luminari S, Eyre TA: The use of frailty assessments in treating older adults with aggressive lymphomas. Br J Haematol 194:677–685, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Palumbo A, Bringhen S, Mateos MV, et al. : Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 125:2068–2074, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Sun CL, Kim H, et al. : Geriatric Assessment-Driven Intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: A randomized clinical trial. JAMA Oncol 7:e214158, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed MR, Rich DQ, Seplaki C, et al. : Primary treatment modification and treatment tolerability among older chemotherapy recipients with advanced cancer. JAMA Netw Open 7:e2356106, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abel GA, Klepin HD: Frailty and the management of hematologic malignancies. Blood 131:515–524, 2018 [DOI] [PubMed] [Google Scholar]
- 26.DuMontier C, Loh KP, Bain PA, et al. : Defining undertreatment and overtreatment in older adults with cancer: A scoping literature review. J Clin Oncol 38:2558–2569, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DuMontier C, Uno H, Hshieh T, et al. : Randomized controlled trial of geriatric consultation versus standard care in older adults with hematologic malignancies. Haematologica 107:1172–1180, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin RJ, Kim SJ, Brown S, et al. : Prospective geriatric assessment and geriatric consultation in CAR T-cell therapy for older patients with lymphoma. Blood Adv 7:3501–3505, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derman BA, Kordas K, Ridgeway J, et al. : Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 3:3488–3498, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muffly L, Pasquini MC, Martens M, et al. : Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood 130:1156–1164, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abel GA, Kim HT, Hantel A, et al. : Fit older adults with advanced myelodysplastic syndromes: Who is most likely to benefit from transplant? Leukemia 35:1166–1175, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura R, Saber W, Martens MJ, et al. : Biologic assignment trial of reduced-intensity hematopoietic cell transplantation based on donor availability in patients 50–75 years of age with advanced myelodysplastic syndrome. J Clin Oncol 39:3328–3339, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DuMontier C, Loh KP, Soto-Perez-de-Celis E, et al. : Decision making in older adults with cancer. J Clin Oncol 39:2164–2174, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman AE, Elias R, Plotkin E, et al. : Models of care in geriatric oncology. J Clin Oncol 39:2195–2204, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puts M, Alqurini N, Strohschein F, et al. : Impact of geriatric assessment and management on quality of life, unplanned hospitalizations, toxicity, and survival for older adults with cancer: The randomized 5C trial. J Clin Oncol 41:847–858, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mian H, McCurdy A, Giri S, et al. : The prevalence and outcomes of frail older adults in clinical trials in multiple myeloma: A systematic review. Blood Cancer J 13:6, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gajra A, Jeune-Smith Y, Fortier S, et al. : The use and knowledge of validated geriatric assessment instruments among US community oncologists. JCO Oncol Pract 18:e1081–e1090, 2022 [DOI] [PubMed] [Google Scholar]
- 38.Jensen-Battaglia M, Sohn M, Consagra W, et al. : Trajectories of physical well-being among adults with acute myeloid leukemia. Blood Adv 10.1182/bloodadvances.2023011804 [epub ahead of print on March 1, 2024] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang LW, Shi Y, Andreadis C, et al. : Association of geriatric measures and global frailty with cognitive decline after allogeneic hematopoietic cell transplantation in older adults. J Geriatr Oncol 14: 101623, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt VR, Wichman C, Koll TT, et al. : Longitudinal changes in cognitive and physical function and health-related quality of life in older adults with acute myeloid leukemia. J Geriatr Oncol 15:101676, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cliff ERS, Mohyuddin GR: Overall survival as a primary end point in multiple myeloma trials. Nat Rev Clin Oncol 19:565–566, 2022 [DOI] [PubMed] [Google Scholar]
- 42.Booth CM, Eisenhauer EA, Gyawali B, et al. : Progression-free survival should not be used as a primary end point for registration of anticancer drugs. J Clin Oncol 41:4968–4972, 2023 [DOI] [PubMed] [Google Scholar]
- 43.Coulson AB, Royle KL, Pawlyn C, et al. : Frailty-adjusted therapy in Transplant Non-Eligible patients with newly diagnosed Multiple Myeloma (FiTNEss (UK-MRA Myeloma XIV trial)): A study protocol for a randomised phase III trial. BMJ Open 12:e056147, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DuMontier C, La J, Bihn J, et al. : More intensive therapy as more effective treatment for frail patients with multiple myeloma [corrected]. Blood Adv 7:6275–6284, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penack O, Peczynski C, Mohty M, et al. : How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv 4:6283–6290, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra A, Preussler JM, Bhatt VR, et al. : Breaking the age barrier: Physicians’ perceptions of candidacy for allogeneic hematopoietic cell transplantation in older adults. Transplant Cell Ther 27:617 e1–e7, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimoni A, Hardan I, Shem-Tov N, et al. : Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: The role of dose intensity. Leukemia 20:322–328, 2006 [DOI] [PubMed] [Google Scholar]
- 48.DiNardo CD, Jonas BA, Pullarkat V, et al. : Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617–629, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Winters AC, Bosma G, Abbott D, et al. : Outcomes are similar after allogeneic hematopoietic stem cell transplant for newly diagnosed acute myeloid leukemia patients who received venetoclax + azacitidine versus intensive chemotherapy. Transplant Cell Ther 28:694.e1–694.e9, 2022 [DOI] [PubMed] [Google Scholar]
- 50.Short NJ, Ong F, Ravandi F, et al. : Impact of type of induction therapy on outcomes in older adults with AML after allogeneic stem cell transplantation. Blood Adv 7:3573–3581, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kröger N, Shahnaz Syed Abd Kadir S, Zabelina T, et al. : Peritransplantation ruxolitinib prevents acute graft-versus-host disease in patients with myelofibrosis undergoing allogenic stem cell transplantation. Biol Blood Marrow Transplant 24:2152–2156, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Ali H, Tsai NC, Synold T, et al. : Peritransplantation ruxolitinib administration is safe and effective in patients with myelofibrosis: A pilot open-label study. Blood Adv 6:1444–1453, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B, Chen L, Zhou J, et al. : Ruxolitinib early administration reduces acute GVHD after alternative donor hematopoietic stem cell transplantation in acute leukemia. Sci Rep 11:8501, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolanos-Meade J, Hamadani M, Wu J, et al. : Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med 388:2338–2348, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Copelan OR, Sanikommu SR, Trivedi JS, et al. : Higher incidence of hemorrhagic cystitis following haploidentical related donor transplantation compared with matched related donor transplantation. Biol Blood Marrow Transplant 25:785–790, 2019 [DOI] [PubMed] [Google Scholar]
- 56.Duléry R, Mohty R, Labopin M, et al. : Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol 3:250–259, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rambaldi B, Kim HT, Reynolds C, et al. : Impaired T- and NK-cell reconstitution after haploidentical HCT with posttransplant cyclophosphamide. Blood Adv 5:352–364, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duléry R, Goudet C, Mannina D, et al. : Reduced post-transplant cyclophosphamide doses in haploidentical hematopoietic cell transplantation for elderly patients with hematological malignancies. Bone Marrow Transplant 58:386–392, 2023 [DOI] [PubMed] [Google Scholar]
- 59.McCurdy SR: Interim results of a phase I/II trial of intermediate-dose post-transplantation cyclophosphamide after reduced intensity conditioned HLA-mismatched bone marrow transplantation for older or unfit patients. Transplant Cell Ther 30:S2–S3, 2024 [Google Scholar]
- 60.Raghunandan S, Qayed M, Watkins BK, et al. : Abatacept for graft versus host disease prophylaxis in patients 60 years and older receiving mismatched unrelated donor transplantation for hematologic malignancies. Bone Marrow Transplant 58:1264–1266, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Artz A: The Composite Health Risk Assessment Model (CHARM) to predict 1-year non-relapse mortality (NRM) among older recipients of allogeneic transplantation: A prospective BMT-CTN study 1704. Blood 142:109, 2023 [Google Scholar]
- 62.Raimondi R, Tosetto A, Oneto R, et al. : Validation of the hematopoietic cell transplantation-specific comorbidity index: A prospective, multicenter GITMO study. Blood 120:1327–1333, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Jayani R, Rosko A, Olin R, et al. : Use of geriatric assessment in hematopoietic cell transplant. J Geriatr Oncol 11:225–236, 2020 [DOI] [PubMed] [Google Scholar]
- 64.Deschler B, Ihorst G, Schnitzler S, et al. : Geriatric assessment and quality of life in older patients considered for allogeneic hematopoietic cell transplantation: A prospective risk factor and serial assessment analysis. Bone Marrow Transplant 53:565–575, 2018 [DOI] [PubMed] [Google Scholar]
- 65.Hurria A, Mohile S, Gajra A, et al. : Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 34:2366–2371, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fried LP, Tangen CM, Walston J, et al. : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Sung AD, Koll T, Gier SH, et al. : Preconditioning frailty phenotype influences survival and relapse for older allogeneic transplantation recipients. Transplant Cell Ther 30:415.e1–415.e16, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]


