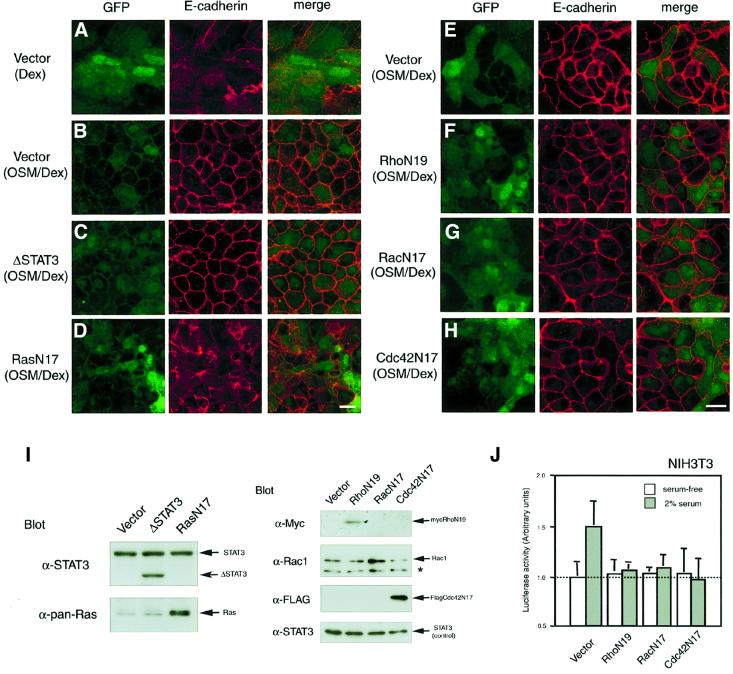
Fig. 4. Effects of dominant-negative forms of signal molecules on OSM-triggered E-cadherin localization at cell–cell junctions. Fetal hepatocytes were infected on day 0 with the pMIG retroviral vector carrying no signaling molecule (A, B and E), ΔSTAT3 (C), RasN17 (D), RhoN19 (tagged with myc) (F), RacN17 (G) or Cdc42N17 (tagged with Flag) (H), and cultured for 6 days with Dex (A) or OSM/Dex (B–H). Viral infection was verified by green fluorescence of GFP (left panels). The localization of E-cadherin in each culture was examined by immunofluorescence analysis using anti-E-cadherin antibody (middle panels). Merged images are shown in the right hand panels. Scale bars: 10 µm. (I) Ectopic expression of dominant-negative molecules in fetal hepatocytes. Cell extracts from each culture were analyzed by western blotting using specific antibodies. ΔSTAT3 is detected as a smaller band than the endogenous STAT3. Expression of RasN17 and RacN17 was verified by augmented expression of Ras and Rac1, respectively (asterisk; non-specific band). Expression of RhoN19 (tagged with myc) and Cdc42N17 (tagged with Flag) was determined by immunoblotting using antibodies against myc and Flag, respectively. Because expression levels of endogenous STAT3 were comparable in each sample, STAT3 was used as an internal control. (J) Inhibitory effect of dominant-negative forms of the Rho family proteins on c-fos transcription in NIH-3T3 cells. To confirm whether retrovirus vectors carrying dominant-negative forms of the Rho family are functional, NIH-3T3 cells infected with these retrovirus vectors were transiently transfected with a reporter plasmid linked to the c-fos promoter and pRL plasmid and cultured with or without 2% serum for 24 h. Luciferase activity was determined using the Dual Luciferase Reporter Assay System.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.