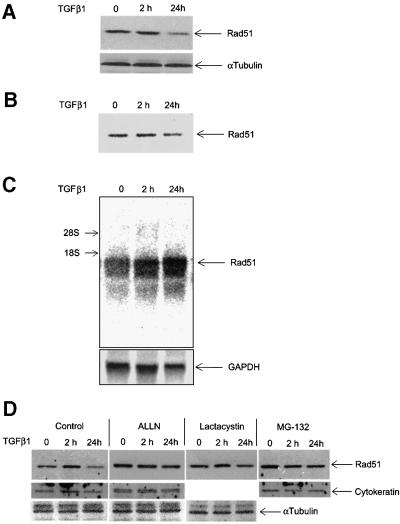
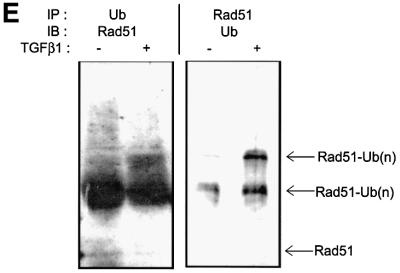
Fig. 5. TGFβ1 inhibits expression of Rad51 protein by affecting its degradation but not its mRNA expression. Expression of endogenous Rad51 protein was evaluated by western blotting (A) and by immunoprecipitation from 35S-labelled cells (B). Mv1Lu cells were treated with TGFβ1 (5 ng/ml) for 2 and 24 h, and total cell lysate was subjected to SDS–PAGE and western blotting (A), or to immunoprecipitation (B) with Rad51-specific antibodies. Migration of Rad51 is shown by arrows. For control of equal loading, expression of α-tubulin was determined using the same cell extracts (for western blotting; A). (C) Rad51 mRNA expression was evaluated by northern blotting of total RNA prepared from cells treated as in (A) and (B), with a human Rad51 probe. Equal loading of RNA was evaluated by re-probing the same membrane with GAPDH probe, as shown. Migration positions for 18S and 28S rRNA are shown. (D) Inhibition of proteasomal degradation by treatment of cells with MG-132 (10 µM), lactacystin (10 µM) or ALLN (12.5 µg/ml) for 6 h (for MG-132) or for 8 h (for lactacystin and ALLN) before cell lysis, blocks TGFβ1-dependent decrease in Rad51 protein expression. Cells were treated with TGFβ1 (5 ng/ml) as indicated. Rad51 protein expression was evaluated by western blotting. Cytokeratin expression was used for normalization of equal loading, as α-tubulin expression in Mv1Lu cells was found to be sensitive to the MG-132 treatment. Migration positions of Rad51, α-tubulin and cytokeratin are shown. (E) TGFβ1 stimulates ubiquitylation of Rad51. Mv1Lu cells were treated with TGFβ1 (5 ng/ml) or not, as indicated. After cell lysis, immunoprecipitation (IP) with anti-ubiquitin or anti-Rad51 antibodies was performed, followed by western blotting (IB) with anti-Rad51 or anti-ubiquitin antibodies, as indicated. Migration positions of unubiquitylated (Rad51) or ubiquitylated Rad51 [Rad51-Ub(n)] are shown. Representative experiments of four (for A, C and D) and three (for B and E) performed, are shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.