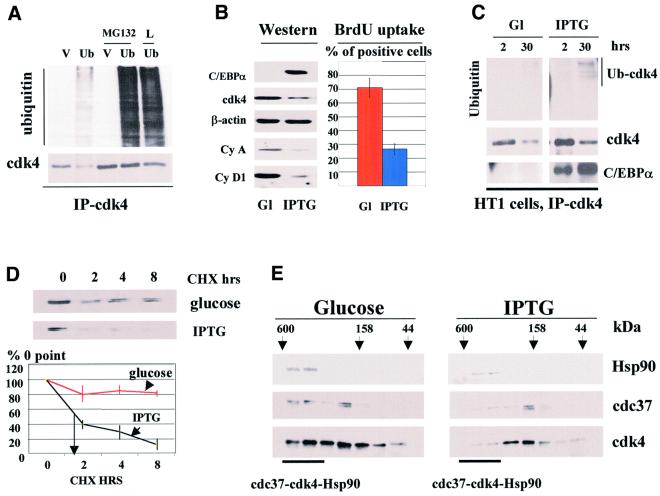
Fig. 6. C/EBPα reduces cdk4–cdc37–Hsp90 complex, induces cdk4–ubiquitin conjugates and shortens the half-life of cdk4 during growth arrest in HT1 cells. (A) Expression of HA-ubiquitin leads to the formation of cdk4–ubiquitin intermediates. COS7 cells were transfected with the empty vector (V, control) or with a plasmid expressing HA-ubiquitin. The next day after transfection, cells were treated with inhibitors of the proteasome MG132 or LlnL (L) or with DMSO (control). Cdk4 was precipitated, and cdk4 immunoprecipitations were analyzed by western blotting with antibodies to the HA-tag (upper) or with antibodies to cdk4 (lower). (B) cdk4 levels are reduced in cells growth arrested by C/EBPα. HT1 cells were synchronized by serum starvation, expression of C/EBPα was induced by IPTG, and cells were plated at low density. Levels of cdk4, cyclin A, cyclin D1 and C/EBPα were determined 30 h after plating in glucose-treated (control) and IPTG-treated cells (Western). β-actin shows the re-probe of the membrane for cdk4. BrdU uptake shows percentage of glucose- and IPTG-treated cells incorporating BrdU at 18 h after plating. (C) cdk4–ubiquitin conjugates are increased in cells arrested by C/EBPα. HT1 cells were synchronized in G1. C/EBPα was induced by IPTG and cells were plated at low density. Cdk4 was precipitated from cells at 2 and 30 h, and cdk4 immunoprecipitations were analyzed by western blotting with antibodies to ubiquitin, cdk4 and C/EBPα. The result shown in the figure represents the re-probe of the same membrane with the aforementioned antibodies. The position of the cdk4–ubiquitin conjugate induced by C/EBPα is shown on the right. (D) The half-life of cdk4 is reduced in growth-arrested HT1 cells. Protein synthesis was blocked by cyclohexamide (CHX) and cdk4 levels were examined by western blotting at 0, 2, 4 and 8 h after CHX addition. The stability of cdk4 was calculated as a ratio to 0 point. A summary of three experiments is shown below. (E) cdk4–cdc37–Hsp90 complex is reduced in C/EBPα growth-arrest cells. Total proteins from control cells (glucose) and from cells arrested by C/EBPα (IPTG) were fractionated by gel filtration, and Hsp90, cdc37 and cdk4 were examined by western blotting with specific antibodies.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.