Abstract
Objective
Metabolic biomarkers are important for the early detection and prognostic assessment of cancer. Nevertheless, a bibliometric analysis examining their research landscape within this domain has not been performed. This study seeks to investigate the current research landscape of metabolic biomarkers related to cancer from 2015 to 2025 and to highlight emerging trends, offering valuable insights for future research directions.
Methods
Articles published from 2015 to 2025 were extracted from the Web of Science database, and an analysis was performed utilizing R software, along with CiteSpace and VOSviewer.We retrieved clinical trials published between 2015 and 2025 from the PubMed database to analyze the clinical progress in this field.
Results
This research encompassed 943 articles in total. Investigations into metabolic biomarkers related to cancer have demonstrated a consistent growth in publications between 2015 and 2023, which was succeeded by a significant surge from 2023 to 2024. The country with the highest number of publications is China, with the United States, the United Kingdom, Japan, and Italy following in that order. Furthermore, China has demonstrated exceptional performance in international collaboration, with the Chinese Academy of Sciences, Shanghai Jiao Tong University, and Zhejiang University emerging as the most prominent collaborative centers. Cancers is recognized as the journal that publishes the greatest quantity of articles within this domain, whereas PLOS One stands out as the journal with the highest citation frequency. Common keywords in the literature include terms such as ‘risk,’ ‘metabolism,’ and ‘breast cancer.’ Research hotspots primarily focus on their application across different cancer types, multi-omics and big data-driven discovery, and the development potential of microbiota-derived markers, and the lag of clinical transformation of metabolic biomarkers, etc.
Conclusion
The vast promise that metabolic biomarkers hold for the diagnosis and treatment of cancer has attracted considerable interest from researchers across the globe. They are anticipated to emerge as a central theme in the future of cancer prevention and therapeutic strategies. This article offers an in-depth analysis of existing research, addresses key challenges in the field, and provides critical insights for future studies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-03887-0.
Keywords: Bibliometric analysis, Metabolic biomarkers, Cancer, Integrated analysis, Metabolic microorganisms
Introduction
Cancer remains one of the most severe threats to global health and is a leading cause of disease-related mortality worldwide, posing a significant barrier to improvements in global life expectancy [1–3]. Recent worldwide data from 2022 reveal around 20 million newly diagnosed cancer cases along with close to 10 million fatalities attributed to cancer. Despite notable progress in socioeconomic development and medical advancements worldwide, the rates of cancer incidence and mortality persist at disturbingly elevated levels, continuing to have a considerable adverse effect on public health globally [4].
In the continuous struggle against cancer, the constraints of existing diagnostic technologies lead to most cases being discovered solely in their later stages, which greatly compromises the efficacy of early therapeutic interventions. This significant shortfall emphasizes the pressing requirement for improved tools to identify, diagnose, and track the disease. Importantly, metabolomics—a thorough analytical methodology that systematically evaluates the complete range of endogenous metabolites within biological systems—has shown considerable promise and expansive applicability in the early diagnosis and tailored treatment of different cancers [5]. To clarify terminology throughout this study, we define “metabolic biomarkers” as a broad category of biological indicators that reflect cancer-associated metabolic alterations, regardless of how they are discovered. In contrast, “metabolomic biomarkers” refer specifically to metabolic markers identified through metabolomics technologies such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). For consistency and simplicity, we primarily use the term “metabolic biomarkers” in a generalized sense, encompassing both traditional and metabolomics-derived indicators.
Metabolomics is a branch of omics science within systems biology that focuses on the comprehensive and quantitative analysis of endogenous metabolites in biological systems. The primary detection methods include nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS), both of which facilitate detailed analyses of metabolites present in cells, tissues, or biological fluids [6]. Significantly, the use of metabolomics within cancer research has resulted in identifying numerous tumor metabolites [7–10] and a wide array of metabolite-derived cancer biomarkers [11–13], several of which have found applications in clinical environments [14, 15]. Currently, metabolic biomarkers are utilized in various aspects of cancer management, including early diagnosis and screening, prognostic assessment, and treatment monitoring. A cohort study conducted prospectively evaluated blood test outcomes from more than 560,000 people, concentrating on nine biomarkers connected to the metabolism of carbohydrates, lipids, and apolipoproteins. Findings revealed that higher concentrations of glucose, total cholesterol, triglycerides, and apolipoprotein A-I are linked to a higher risk of head and neck cancer (HNC), especially squamous cell carcinoma. These findings provide new high-quality evidence for the early involvement of carbohydrate and lipid metabolism in the human carcinogenic process [16]. Furthermore, a connected investigation highlighted the essential function of the L-arginine/nitric oxide (L-ARG/NO) pathway in the onset and progression of ovarian cancer by conducting an in-depth analysis of gene expression patterns in ovarian cancer tissues and the levels of metabolites in the bloodstream. Additionally, the research suggested that the ratio of symmetric dimethylarginine (SDMA) to arginine in serum may act as a promising liquid biopsy biomarker, offering fresh perspectives and pathways for the early identification and diagnosis of ovarian cancer [17].
In summary, although many potential metabolic biomarkers have been identified with promising applications in oncology, most of these biomarkers have not undergone comprehensive clinical validation. There is an urgent need for large-scale, multi-center studies to confirm their efficacy and reliability. Furthermore, the clinical translation of metabolic biomarkers faces numerous challenges that must be addressed from technical, methodological, and biological perspectives. Alterations in lipid metabolism are strongly associated with the development and advancement of tumors, while fluctuations in blood lipid levels may influence the prognosis of these tumors [18]. Recent studies indicate that biomarkers related to lipid metabolism, especially HDL-C, TC, and ApoA1, could act as possible prognostic indicators for survival in cancer patients, with their concentrations possibly facilitating the identification of individuals at high risk [19]. Although there has been a continuous rise in scholarly articles examining the link between metabolic biomarkers and cancer, a thorough bibliometric analysis in this area is still lacking. As a result, achieving a complete understanding of overall research patterns, main areas of focus, and knowledge advancement trajectories remains difficult.
Bibliometrics is a discipline that systematically studies the quantitative characteristics of academic literature, including aspects such as publication volume, quality, citation patterns, and development trends. Utilizing mathematical and statistical techniques to examine books, scholarly articles, and various types of academic discourse, bibliometrics uncovers new research frontiers, identifies emerging hotspots, and highlights evolutionary trends. Some bibliometric studies have analyzed the research status of cancer biomarkers. For example, Xu et al.conducted a bibliometric analysis of cervical cancer screening biomarkers, focusing on the publication trends and hotspots of screening biomarkers [20]. Similarly, Yuan et al.used co-citation and keyword clustering techniques to explore biomarkers related to cancer immunotherapy [21]. However, there are more and more studies on metabolic biomarkers of cancer, but comprehensive bibliometric analysis of the evolution of this field is still insufficient. Importantly, there are still some unanswered questions : How has the integration of multi-omics approaches influenced the discovery of metabolic biomarkers ? Which types of cancer are understudied in this field ? How does the global cooperation network affect the progress of this research field? Solving these problems through bibliometric methods is crucial for systematically identifying research gaps, highlighting emerging trends, and guiding future research.In this study, we aim to capture the latest developments and emerging topics. Considering that there are few studies before 2015 and the inclusion of earlier publications will weaken the focus on recent methodological progress and research priorities, especially research related to multi-omics integration and big data methods. Therefore, we conducted an in-depth bibliometric analysis of literature related to cancer and metabolic biomarkers published between 2015 and 2025, utilizing analytical tools such as R (Bibliometrix package), CiteSpace, and VOSviewer. The main goal of our study is to pinpoint significant research hotspots and offer important insights for upcoming investigations.
Materials and methods
Data collection
The data utilized in this study were sourced from the Web of Science (Shandong University of Traditional Chinese Medicine Edition) and PubMed database on March 26, 2025. The search strategy is shown in Annex 1. To ensure data accuracy, we performed deduplication in multiple stages. First, the export was processed to remove redundant records by comparing titles, DOIs, and author lists. Records with identical titles and DOIs were considered duplicates and excluded, and selected articles are stored in a plain text format. We created and included a PRISMA-like flowchart (shown in Annex 2) to clearly document the process of article identification, screening, and inclusion. Furthermore, the references cited have been exported as comprehensive records. The clinical trial results are exported in PubMed format.
Data analysis
This research utilizes Origin 2025 software to examine annual trends in publication data. It also incorporates R software (version 4.3.2) alongside the Bibliometrix package (version 4.0), as well as VOSviewer (version 1.6.2) [22] and CiteSpace (version 6.3.1) [23] for the analysis and visualization of bibliometric information. The selected tools were deliberately chosen to guarantee the precision and dependability of the data extraction processes and analytical approaches. VOSviewer was essential in generating several visual depictions, including co-authorship networks categorized by country and institution, assessments of co-citation, and networks demonstrating the co-occurrence of keywords. In developing the co-authorship networks, our focus was solely on those countries and institutions that generated five or more publications within the scope of our analysis. Using VOSviewer's thesaurus function and manual review, the organization names were standardized to address changes ( e.g., ' Univ California SF ' vs ' Univ Calif San Francisco ' ). For multi-authored papers with multiple institutional affiliations, all listed institutions were included in the institutional collaboration analysis to capture the full scope of collaboration networks. The co-citation evaluation specifically concentrated on documents that accumulated 115 or more citations. For the analysis of keyword co-occurrence, only terms found in at least eight publications were included. Frequently appearing keywords (like ‘cancer’ and ‘metabolic biomarkers’) were omitted to prevent their regular presence in search queries from skewing the analytical results and obstructing the detection of other significant keywords. Data regarding the impact factor (IF) of journals was sourced from the 2023 Journal Citation Reports (JCR). We used different visualization types for specific analytical purposes. Collaboration maps show key countries and institutions and their partnership strengths. Co-citation networks identify influential journals. Citation burst analysis highlights emerging research. Keyword co-occurrence reveals thematic clusters, while trend topics trace topic evolution over time. These visual tools provide complementary insights and improve reproducibility. Utilizing these analytical approaches and methodologies, this research offers an in-depth and thorough viewpoint on the trends and dynamics related to cancer research and metabolic biomarkers.
Results
General landscape of included documents on metabolic biomarkers and cancer
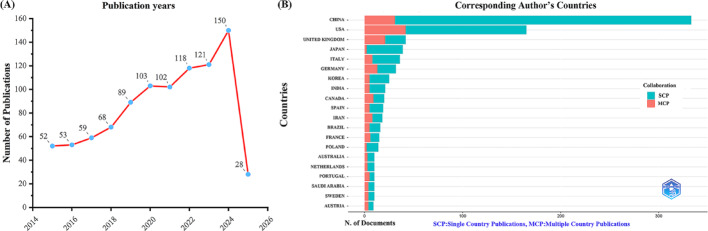
Following a thorough examination of the WoS database, we discovered 943 publications concerning metabolic biomarkers and cancer. As illustrated in Fig. 1A, this area has shown a consistent upward trajectory in the number of publications over the last ten years. The publication volume in this field has steadily increased from 2015 to 2023, followed by a marked surge in 2023–2024. This acceleration suggests growing academic interest and possibly reflects the advancement of metabolomics techniques and their expanded application in oncology. To date, 943 articles have been published in this field.
Fig. 1.
Annual publication trends concerning the association between metabolic biomarkers and cancer from 2015 to 2025. A Trends in yearly publications. B Patterns of geographic distribution and collaboration among corresponding authors
An examination of the institutions affiliated with the corresponding authors indicates that China (333 articles) is at the forefront of research output, followed by the United States (165 articles), the United Kingdom (42 articles), Japan (39 articles), and Italy (36 articles). These results highlight the significant contributions of both the United States and China in researching metabolic biomarkers and cancer. Notably, when examining the top five countries based on publication volume, the rates of international collaboration for Japan and China (5.1% and 9.3%, respectively) are significantly less than those of Italy (22.2%), the United Kingdom (50%), and the United States (25.5%). This indicates that international collaboration is less prevalent for both China and Japan within this field, as demonstrated in Fig. 1B and Table 1. In addition, most of the top 10 countries have sound biomedical research infrastructure and strong biomedical research capabilities. High-income countries have shown more international cooperation and have achieved fruitful results in cancer and metabolomics research. Furthermore, Fig. 2A illustrates that China has the broadest collaborative network with other countries regarding research on metabolic biomarkers and cancer. The collaboration map further reveals key centers of cooperation, including the Chinese Academy of Sciences (n = 30), Zhejiang University (n = 22), and Shanghai Jiao Tong University (n = 22) (Fig. 2B and Table 2).
Table 1.
Most relevant countries by corresponding authors of the relationship between metabolic biomarkers and cancer
| Country | Articles | SCP | MCP | Frep % | MCP_Ratio % |
|---|---|---|---|---|---|
| CHINA | 333 | 302 | 31 | 35.3 | 9.3 |
| USA | 165 | 123 | 42 | 17.5 | 25.5 |
| UNITED KINGDOM | 42 | 21 | 21 | 4.5 | 50 |
| JAPAN | 39 | 37 | 2 | 4.1 | 5.1 |
| ITALY | 36 | 28 | 8 | 3.8 | 22.2 |
| GERMANY | 32 | 19 | 13 | 3.4 | 40.6 |
| KOREA | 25 | 20 | 5 | 2.7 | 20 |
| INDIA | 21 | 16 | 5 | 2.2 | 23.8 |
| CANADA | 20 | 11 | 9 | 2.1 | 45 |
| SPAIN | 19 | 14 | 5 | 2 | 26.3 |
| IRAN | 18 | 10 | 8 | 1.9 | 44.4 |
| BRAZIL | 16 | 11 | 5 | 1.7 | 31.3 |
| FRANCE | 15 | 9 | 6 | 1.6 | 40 |
| POLAND | 14 | 12 | 2 | 1.5 | 14.3 |
| AUSTRALIA | 10 | 7 | 3 | 1.1 | 30 |
| NETHERLANDS | 10 | 7 | 3 | 1.1 | 30 |
| PORTUGAL | 10 | 5 | 5 | 1.1 | 50 |
| SAUDI ARABIA | 10 | 6 | 4 | 1.1 | 40 |
| SWEDEN | 10 | 6 | 4 | 1.1 | 40 |
| AUSTRIA | 9 | 5 | 4 | 1 | 44.4 |
MCP multiple-country publication, SCP single-country publication
Fig. 2.
Maps illustrating the collaborations of countries/regions and institutions engaged in studies regarding the connection between metabolic biomarkers and cancer from 2015 to 2025. A Global collaboration between nations. B Collaboration among various research institutions
Table 2.
Most relevant affiliations of the relationship between metabolic biomarkers and cancer
| Affiliations | Articles |
|---|---|
| Chinese Academy of Sciences | 30 |
| Shanghai Jiao Tong University | 22 |
| Zhejiang University | 22 |
| Chinese Academy of Medical Sciences | 21 |
| Fudan University | 16 |
| Harbin Medical University | 15 |
| Sun Yat-sen University | 15 |
| UT MD Anderson Cancer Center | 14 |
| Zhengzhou University | 14 |
| China Pharmaceutical University | 13 |
| Harvard Medical School | 13 |
| National Cancer Institute | 13 |
| University of California, San Francisco | 13 |
| Fujian Medical University | 12 |
| Soochow University | 12 |
| Imperial College London | 11 |
| Keio University | 11 |
| Nanjing University | 11 |
| Shandong University | 11 |
| Southern Medical University | 11 |
| German Cancer Research Center | 10 |
| Karolinska Institute | 10 |
| Nanjing Medical University | 10 |
| University of Chinese Academy of Sciences | 10 |
| Capital Medical University | 9 |
Journals and co-cited journals
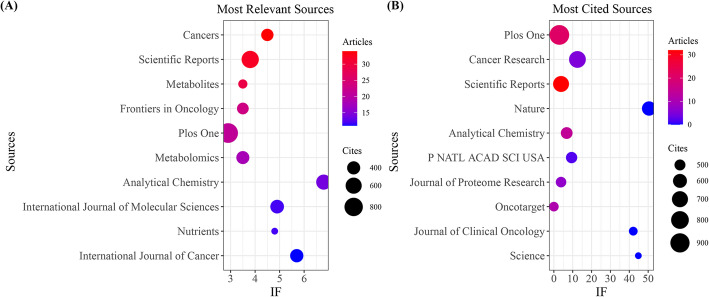
The analysis of journals with the greatest number of publications and citations related to cancer and metabolic biomarkers was conducted using R software (version 4.3.2), in conjunction with the Bibliometrix and ggplot2 packages. Furthermore, VOSviewer (version 1.6.2) was employed for a co-citation analysis of those journals. The results indicate that 943 articles were published in 477 distinct academic journals (Annex 3).
As illustrated in Fig. 3A and Table 3, the journal Cancers (n = 34, IF = 4.5) has the largest number of published articles, followed by Scientific Reports (n = 32, IF = 3.8), Metabolites (n = 29, IF = 3.5), Frontiers in Oncology (n = 23, IF = 3.5), and PLOS One (n = 21, IF = 2.9).Top 5 journals share traits such as interdisciplinary focus, moderate-to-high impact factors, and openness to omics-based cancer research with strong data visualization.
Fig. 3.
Journals featuring the most publications and citations concerning metabolic biomarkers and cancer. A Journals organized by total citation volume. B Journals listed by the quantity of published articles
Table 3.
Top 10 journals with the most published articles
| Sources | Articles | Cites | IF |
|---|---|---|---|
| Cancers | 34 | 360 | 4.5 |
| Scientific Reports | 32 | 695 | 3.8 |
| Metabolites | 29 | 253 | 3.5 |
| Frontiers in Oncology | 23 | 333 | 3.5 |
| Plos One | 21 | 936 | 2.9 |
| Metabolomics | 18 | 395 | 3.5 |
| Analytical Chemistry | 14 | 522 | 6.8 |
| International Journal of Molecular Sciences | 12 | 430 | 4.9 |
| Nutrients | 12 | 228 | 4.8 |
| International Journal of Cancer | 11 | 410 | 5.7 |
Figure 3B and Table 4 illustrate the most cited journals, with PLOS One (n = 936, IF = 2.9) ranking first, followed by Cancer Research (n = 741, IF = 12.5), Scientific Reports (n = 695, IF = 3.8), Nature (n = 615, IF = 50.5), and Analytical Chemistry (n = 522, IF = 6.8).
Table 4.
Top 10 journals with the most cited journals
| Sources | Cites | Articles | IF |
|---|---|---|---|
| Plos One | 936 | 21 | 2.9 |
| Cancer Research | 741 | 5 | 12.5 |
| Scientific Reports | 695 | 32 | 3.8 |
| Nature | 615 | 0 | 50.5 |
| Analytical Chemistry | 522 | 14 | 6.8 |
| P NATL ACAD SCI USA | 509 | 2 | 9.4 |
| Journal of Proteome Research | 490 | 7 | 3.8 |
| Oncotarget | 472 | 11 | 0 |
| Journal of Clinical Oncology | 460 | 0 | 42.1 |
| Science | 448 | 0 | 44.8 |
The analysis of journal citations through visualization (Fig. 4) reveals that PLOS ONE, Cancer Research, and Scientific Reports are among the most influential journals in this area. These results highlight the important contributions of PLOS ONE and Cancer Research to the study of metabolic biomarkers and cancer research. Notably, high-impact journals such as Nature publish relatively few articles on this specific topic but achieve disproportionately high citation counts. This reflects their selective editorial policies and their emphasis on publishing landmark studies with broad, long-term influence, a common phenomenon in bibliometric patterns across disciplines.
Fig. 4.
Co-cited journals contributing to research on the relationship between metabolic biomarkers and cancer
Most cited references and reference burst
Using the Bibliometrix package in R, we discovered the 20 articles most often cited regarding metabolic biomarkers linked to cancer, each garnering over 115 citations and published in 18 different academic journals (Table 5). This finding suggests that considerable theoretical advancements have yet to be accomplished in this area. Notably, the journals publishing these highly cited articles do not dominate the landscape. The most cited articles include ‘Metagenomic and Metabolomic Analyses Reveal Distinct Stage-Specific Phenotypes of the Gut Microbiota in Colorectal Cancer’ and ‘Lipid Desaturation as a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells’ which primarily provide a comprehensive description of metabolic biomarkers and their relationship with cancer.Among the top-cited references, several stand out due to methodological innovations or translational significance. For instance, Yachida et al. revealed the stage-specific microbiota characteristics of colorectal cancer using fecal metagenomics and metabolomics studies, providing a blueprint for the discovery of microbiota-based biomarkers [24]. Similarly, Li et al. introduced lipid desaturation as a functional marker of ovarian cancer stemness, establishing a metabolic vulnerability with therapeutic implications [25]. The high citation counts of these studies reflect their dual impact—advancing mechanistic understanding while offering clinically actionable insights.
Table 5.
Top 20 cited references related to metabolic biomarkers and cancer
| Paper | DOI | Total citations | TC per year | Normalized TC |
|---|---|---|---|---|
| YACHIDA S, 2019, NAT MED | 10.1038/s41591-019-0458-7 | 833 | 119.00 | 20.84 |
| LI JJ, 2017, CELL STEM CELL | 10.1016/j.stem.2016.11.004 | 426 | 47.33 | 10.41 |
| LEE YM, 2017, NUTRIENTS | 10.3390/nu9101089 | 254 | 28.22 | 6.21 |
| MILOUSHEV VZ, 2018, CANCER RES | 10.1158/0008-5472.CAN-18-0221 | 183 | 22.88 | 4.92 |
| MAYERLE J, 2018, GUT | 10.1136/gutjnl-2016-312432 | 182 | 22.75 | 4.90 |
| BUERGEL T, 2022, NAT MED | 10.1038/s41591-022-01980-3 | 174 | 43.50 | 13.56 |
| SERRA-MAJEM L, 2019, MOL ASPECTS MED | 10.1016/j.mam.2019.06.001 | 158 | 22.57 | 3.95 |
| JULKUNEN H, 2023, NAT COMMUN | 10.1038/s41467-023-36231-7 | 152 | 50.67 | 21.46 |
| DASARI S, 2015, CLIN CHIM ACTA | 10.1016/j.cca.2015.03.005 | 145 | 13.18 | 3.84 |
| ISHIKAWA S, 2016, SCI REP-UK | 10.1038/srep31520 | 143 | 14.30 | 4.97 |
| VAN DER SCHEE MP, 2015, CHEST | 10.1378/chest.14-0781 | 141 | 12.82 | 3.74 |
| ZHANG JS, 2019, REPROD BIOL ENDOCRIN | 10.1186/s12958-019-0509-4 | 139 | 19.86 | 3.48 |
| MONDUL AM, 2015, INT J CANCER | 10.1002/ijc.29576 | 138 | 12.55 | 3.66 |
| HOLSCHER HD, 2018, J NUTR | 10.1093/jn/nxy004 | 131 | 16.38 | 3.53 |
| COKER OO, 2022, MICROBIOME | 10.1186/s40168-021-01208-5 | 129 | 32.25 | 10.05 |
| ELLULU MS, 2015, DRUG DES DEV THER | 10.2147/DDDT.S83144 | 129 | 11.73 | 3.42 |
| KHAW KT, 2018, BMJ OPEN | 10.1136/bmjopen-2017-020167 | 125 | 15.63 | 3.36 |
| SONG P, 2020, NAT COMMUN | 10.1038/s41467-020-14664-8 | 121 | 20.17 | 5.38 |
| DINGES SS, 2019, NAT REV UROL | 10.1038/s41585-019-0185-3 | 120 | 17.14 | 3.00 |
In order to identify important citation bursts associated with the link between metabolic biomarkers and cancer, we employed CiteSpace to find 30 references that displayed substantial citation bursts according to defined criteria (top 25; status count: 2; minimum duration: 2). Out of these, 25 references are illustrated in Fig. 5. The reference titled ‘Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries’ had the highest citation strength of 14.63, followed by ‘Metabolomics in Cancer Research and Emerging Applications in Clinical Oncology’ and ‘MetaboAnalyst 5.0: Narrowing the Gap Between Raw Spectra and Functional Insights’ with strengths of 7.39 and 6.73, respectively.It is noteworthy that three major citation surges include ‘Targeting Cancer Metabolism in the Era of Precision Oncology’, ‘Cancer Metabolomic Markers in Urine: Evidence, Techniques, and Recommendations’, and ‘Hallmarks of Cancer: New Dimensions’. Each of these works provides profound insights into the field.
Fig. 5.
Top 25 references exhibiting the strongest citation bursts in research on the relationship between metabolic biomarkers and cancer
To gain a deeper insight into the forefront and key areas of research, we matched the DOIs of the 25 articles referenced in Fig. 5 with the titles found in Annex 4. The findings show that 25% of the references concentrate on the mechanisms underlying metabolic reprogramming in cancer cells, such as heightened glycolysis, irregular amino acid metabolism, and modified lipid metabolism. Another 25% pertain to the application of metabolic biomarkers in cancer diagnosis, highlighting their significant value in early cancer screening and classification. Additionally, 20% of the citations discuss the relationship between the gut microbiota and its metabolites with cancer, exploring the impact of microbial metabolites on host cancer occurrence and progression. 15% of the citations concentrate on metabolic interventions, including strategies and effects in cancer treatment. The remaining 15% focus on multi-omics integration and cancer metabolism research driven by big data.
Keyword clusters and evolution
The analysis of keyword clustering successfully reveals significant research focal points and developmental trajectories within different academic fields. In this study, we utilized VOSviewer to compile a total of 4,711 keywords from relevant literature. As illustrated in Table 6, there are 20 keywords with more than 40 occurrences; among these, ‘diagnosis’ ranks highest with 103 instances, followed by ‘risk’ (n = 94), ‘metabolism’ (n = 89), ‘breast cancer’ (n = 87), ‘expression’ (n = 76), ‘inflammation’ (n = 73), ‘cell’ (n = 72), and ‘obesity’ (n = 67).
Table 6.
Top 20 keywords related to metabolic biomarkers and cancer
| Rank | Key words | Occurrences |
|---|---|---|
| 1 | Diagnosis | 103 |
| 2 | Risk | 94 |
| 3 | Metabolism | 89 |
| 4 | Breast cancer | 87 |
| 5 | Expression | 76 |
| 6 | Inflammation | 73 |
| 7 | Cell | 72 |
| 8 | Obesity | 67 |
| 9 | Serum | 65 |
| 10 | Metabolite | 58 |
| 11 | Mass spectrometry | 56 |
| 12 | Prostate cancer | 55 |
| 13 | Colorectal cancer | 53 |
| 14 | Lung cancer | 52 |
| 15 | Prognosis | 52 |
| 16 | Survival | 49 |
| 17 | Identification | 47 |
| 18 | Insulin resistance | 47 |
| 19 | Pathway | 42 |
| 20 | Association | 41 |
In addition, we identified 183 keywords that meet the criterion of appearing at least eight times. These keywords were used to generate a keyword clustering diagram (Fig. 6). This visualization illustrates five distinct clusters, each represented by a different color.
Fig. 6.
Keyword co-occurrence map of publications on the relationship between metabolic biomarkers and cancer
The Cluster of Metabolic Inflammation and Cancer Risk (Red Dot) comprises 55 keywords, including ‘risk,’ ‘inflammation,’ ‘obesity,’ ‘insulin resistance,’ and ‘association.’ The Cluster of Metabolic Biomarkers and Cancer (Green Dot) consists of 50 keywords, such as ‘diagnosis,’ ‘breast cancer,’ ‘serum,’ ‘metabolite,’ and ‘mass spectrometry.’ The Cluster of Metabolic Biomarkers and Cancer Regulation (Blue Dot) includes 47 keywords related to ‘metabolism,’ ‘expression,’ ‘cell,’ and ‘pathway.’ The Cluster of Metabolic Biomarkers and The Diagnosis and Treatment of Lung Cancer (Yellow Dot) is made up of 20 keywords, including ‘lung cancer,’ ‘prognosis,’ ‘survival,’ and ‘protein.’ Finally, the Cluster of Biomarkers and The Diagnosis and Treatment of Colorectal Cancer (Purple Dot) contains 5 keywords, such as ‘colorectal cancer,’ ‘Alzheimer’s disease,’ ‘colon cancer,’ ‘metformin,’ and ‘system’ (Annex 5). Notably, although the cluster related to “intestinal microbiota” appears in the keyword co-occurrence network, its direct linkage with terms such as “immunotherapy” or “immune response” is limited. This suggests that the intersection between microbial metabolic processes and cancer immunotherapy remains underrepresented in current research. Given the growing evidence of the gut microbiota’s influence on immune modulation and treatment efficacy [26–28], this gap points to a promising direction for future investigations into microbiota-informed precision oncology.
Additionally, we utilized the Bibliometrix package within R to construct a trend topic map. This map serves as an essential tool for identifying the evolution of research themes within a specific field, allowing us to examine the developments and changes in research over time. By analyzing the trend topic map illustrated in Fig. 7, we identified key areas of research and the evolutionary trajectories at different time intervals within the field. Our findings indicate that current research primarily focuses on the use of metabolic biomarkers for cancer diagnosis and risk assessment, particularly when combined with metabolomics technology.
Fig. 7.
Trend topics on the relationship between metabolic biomarkers and cancer
Clinical progress analysis
A total of 39 clinical trials were retrieved from the PubMed database (Annex 6). Clinical trials in this field mainly focus on three aspects (1). Modulation of Metabolic Biomarkers Through Lifestyle Interventions (2). Identification of Metabolic Biomarkers for Cancer Risk and Prognosis (3). Pharmacological or Nutraceutical Modulation of Metabolic Pathways.
Discussion
General information
In this research, we created a comprehensive dataset comprising 943 articles published between 2015 and 2025. Through the analysis of publication trends, we observed a consistent rise in academic output related to metabolic biomarkers and their association with cancer. As illustrated in Fig. 1A, the number of publications has steadily increased, with a particularly notable surge between 2023 and 2024. Regarding metabolic biomarkers and cancer research, China has positioned itself as a frontrunner, boasting a considerably larger volume of published studies. Regarding publication venues, Table 3 and Fig. 3A indicate that among the 477 journals publishing these 943 articles, Cancers (n = 34), Scientific Reports (n = 32), Metabolites (n = 29), Frontiers in Oncology (n = 23), and PLOS One (n = 21) emerged as the most active outlets. Their prominence reflects not only the oncology research community’s strong interest in the topic but also the growing recognition of metabolic biomarkers as clinically relevant tools.
Hotspots and development trends
An in-depth examination of frequently cited sources, reference surges, keyword clustering, and emerging themes uncovers the primary research hotspots and frontiers concerning cancer metabolic biomarkers. Our findings are consistent with previous cancer research bibliometric studies, which have also found the dominance of certain countries and journals. However, by focusing specifically on the application of metabolic biomarkers across various cancer types, research on cancer metabolic biomarkers driven by multi-omics integration and big data, the application and development potential of microbial metabolic biomarkers in cancer, and the lag of clinical transformation of metabolic biomarkers, etc.
The application of metabolic biomarkers across various cancer types
As shown in Table 6, cancers such as breast cancer ( n = 87 ), colorectal cancer ( n = 53 ), lung cancer ( n = 52 ) and prostate cancer ( n = 55 ) have the highest frequency of occurrence as keywords in the literature, which also supports our focus on these cancer types. First, we focus on the utilization of metabolic biomarkers to study different types of cancer. Our keyword clustering analysis indicates that breast cancer, prostate cancer, colorectal cancer, and lung cancer are the most extensively researched cancer types in this field. Notably, metabolic biomarkers have garnered significant attention in various studies due to their importance in early cancer diagnosis, predicting treatment responses, and assessing prognosis. For instance, recent findings underscore the immense potential of metabolic biomarkers derived from extracellular vesicles (EVs) in the diagnosis and prognosis of prostate cancer (PCa). This highlights the critical role of metabolomics as a biomarker discovery platform, paving a new pathway for the early identification and risk assessment of PCa. Future research should prioritize the practical applications of EV-derived metabolic biomarkers in predicting PCa risk and tracking treatment responses.Establishing standardized methods for the isolation and analysis of extracellular vesicles (EVs) is crucial for advancing this field towards clinical applications [29–31]. A prospective cohort study based on population data performed a nuclear magnetic resonance (NMR) metabolomics analysis of initial plasma samples taken from 91,472 individuals in the UK Biobank, uncovering 109 metabolites that demonstrate a significant association with the risk of lung cancer. Among these, glycoprotein acetylation demonstrated a positive correlation with the risk of lung cancer, whereas various metabolites and lipoprotein subfractions exhibited negative correlations. This research represents the inaugural systematic evaluation of the influence of metabolic biomarkers on lung cancer risk within a substantial cohort study. This effort deepens our comprehension of the metabolic processes linked to lung cancer and may suggest novel biomarkers for lung cancer screening [32]. Moreover, numerous studies have emphasized the significant potential of biomarkers related to amino acid and lipid metabolism in the diagnosis, prediction, subtype classification, and treatment response expectations of breast cancer. The observed prominence of lipid metabolism biomarkers aligns with known biological mechanisms, as lipid metabolic reprogramming is a hallmark of cancer progression and prognosis. Additionally, lipidomics methods are relatively mature and accessible, facilitating their frequent use in biomarker discovery studies.However, for these biomarkers to be successfully applied in the clinical management of cancer, it is crucial to conduct further validation through large-scale cohort studies and develop standardized analytical techniques [33–37]. Building on previous work, a recent study utilized LEfSe analysis, random forest modeling, co-occurrence network analysis, and other advanced technologies to identify a set of global microbial and metabolic biomarkers associated with colorectal cancer (CRC). These biomarkers are considered strong candidates for aiding in the early diagnosis and treatment methods of CRC. However, more research is needed to elucidate their underlying mechanisms [38]. These trends are also aligned with high-impact references in Table 5, such as Yachida et al. (2019, Nat Med), which focused on colorectal cancer and microbial metabolism. Despite the identification of metabolic biomarkers across major cancer types, limited effort has been made to compare their diagnostic or prognostic value across different cancer stages or populations. Future research should aim to conduct cross-cancer, multi-cohort validation studies to determine the universality or specificity of certain biomarkers. Moreover, there is a pressing need for harmonized methodologies in metabolite quantification to enable more reproducible clinical applications.
Common cancers, including breast cancer, prostate cancer, colorectal cancer, and lung cancer, have become key focal points of research. While other types, such as gastric or pancreatic cancer, appear less frequently. This underscores potential underexplored areas for future investigation.This predominance may reflect both disease burden and the relative ease of biomarker validation for these cancer types, but it also highlights underexplored opportunities in less-studied cancers.
Research on cancer metabolic biomarkers driven by multi-omics integration and big data
As metabolomics datasets grow more complex, traditional analyses face challenges in identifying clinically relevant biomarkers. Integrating multi-omics data with artificial intelligence has emerged as a promising strategy to address these challenges by uncovering complex biological relationships and enhancing prediction accuracy.
First, the systematic integration of multi-omics data is a key strategy for comprehensively understanding the biological essence of cancer and elucidating its molecular pathogenesis. This approach enables extensive investigation of the molecular properties of cancer from various perspectives, including genomics, transcriptomics, proteomics, and metabolomics. Consequently, it provides new insights and theoretical foundations for the development of targeted cancer therapies [39]. As artificial intelligence technologies continue to advance, especially with the extensive use of machine learning and deep learning algorithms, researchers are increasingly able to investigate intricate patterns and possible trends in metabolomics data that conventional statistical techniques may miss. The combination of multi-omics data and the powerful analytical capabilities of artificial intelligence makes it possible to more accurately identify individual differences among cancer patients [40]. Numerous studies have conducted in-depth investigations into breast cancer by integrating metabolomics with other omics datasets and employing machine learning methods. Several studies have leveraged single-cell transcriptomics and metabolomics to identify metabolic biomarkers for early breast cancer detection and treatment response prediction. Analysis of transcriptomic profiles from cancerous and normal breast tissues revealed a strong link between nucleotide metabolism and regulatory T cell activation in the tumor microenvironment. Using machine learning and absolute quantification metabolomics, researchers developed a four-metabolite model that accurately predicted neoadjuvant chemotherapy responses in triple-negative breast cancer (TNBC) across multiple cohorts [41]. Similar studies have detected metabolic changes in triple-negative breast cancer (TNBC) using metabolomics techniques and explored potential molecular mechanisms through integrative analysis with transcriptomics. Research has uncovered metabolic biomarkers that could be beneficial for diagnosing and screening TNBC, thus offering significant validation for studies conducted in this area [42]. Nguyen Ky Anh and colleagues employed non-targeted metabolomics and lipidomics combined with machine learning models to identify and confirm metabolism-related biomarkers. This strategy highlights the advantages of integrating multi-omics data with machine learning to enhance the detection and validation capabilities of new biomarkers in breast cancer screening [43]. Furthermore, the results indicate that the combination of salivary metabolomics with machine learning methods demonstrates high accuracy and adaptability in identifying colorectal cancer [44]. As seen in Fig. 5, references such as “Targeting Cancer Metabolism in the Era of Precision Oncology” [45]and “MetaboAnalyst 5.0” [46] reflect the rising impact of AI-based, integrative omics approaches in this field.
The integration of multi-omics and artificial intelligence technologies is driving cancer research towards greater accuracy and depth. To ensure the responsible and effective use of metabolic biomarkers in cancer research and clinical applications through artificial intelligence, various challenges still need to be addressed. These challenges include data integration and standardization, algorithm interpretability, data security, ethical issues, as well as the validation of models and their translational application in clinical practice.Their integration into metabolic biomarker studies remains relatively limited. This suggests that while these trends are well-recognized, actual implementation is still developing, highlighting a gap between conceptual enthusiasm and routine application.
The application and development potential of microbial metabolic biomarkers in cancer
The metabolic axis between the microbiota and the host involves complex interactions, primarily stemming from the gut microbiome and facilitated by various metabolic products. These metabolic products act as key signaling molecules that influence the host’s metabolic activities, immune responses, physiological functions, and disease progression [47]. Under physiological conditions, these products help regulate immune function and assist in the elimination of abnormal cells. Conversely, when there is an imbalance in these metabolic products, the host’s immune system may struggle to recognize and eradicate tumor cells in the early stages of cancer development, ultimately leading to tumor progression.
The gut microbiota produces various metabolites that may influence gene expression, cell cycle processes, apoptosis, and immune system activity. These interactions have been associated with cancer progression, although much of the evidence remains correlative and warrants further mechanistic investigation [48]. For example, metabolites produced by the microbiota play a vital role in the regulation of anti-tumor immunity and can influence the effectiveness of both immunotherapy and chemotherapy. Studies have suggested that the gut microbiota-derived metabolite butyrate may enhance the efficacy of certain cancer treatments by modulating CD8+ T cell responses. These findings suggest the potential of microbial metabolites as biomarkers or adjuvants in cancer therapy [49]. Furthermore, another study found that the short-chain fatty acid (SCFA)-GPR43 axis is vital in preventing intestinal bacterial invasion, chronic inflammation, and associated cancers [50]. Furthermore, some studies have reported associations between gut microbiota-derived metabolites and breast cancer risk, though causality remains to be determined [51]. Chinese researchers carried out a study that examined blood samples from 107 patients diagnosed with breast cancer and 107 healthy controls matched by age, employing 16 S rRNA gene sequencing technology. They also evaluated the levels of microbial metabolites through untargeted metabolomics. The results indicate that a combined diagnostic approach utilizing microbiota and gut metabolites may act as a non-invasive biomarker for breast cancer; nevertheless, additional validation with larger sample sizes and multi-center trials is required [52].
In summary, there has been a growing body of research in this field, as evidenced by the sharp increase in citations (Fig. 5). Notably, the number of articles focusing on microorganisms has risen significantly, and keyword co-occurrence analysis (Fig. 6) highlights terms such as ‘inflammation’, ‘intestinal microbiota’, and ‘gut microbiota’ as key phrases. However, the relationship between microbiome-derived metabolites and cancer remains complex and multifaceted, with many studies still in the preliminary stages. While interest in exploring these interactions within the context of cancer therapy is increasing, further mechanistic studies and well-designed clinical trials are necessary to determine whether and how modulation of microbial metabolites can enhance treatment outcomes.
Niche findings: emerging trends and unmet challenges
In addition to general trends such as publication growth and international collaboration patterns, our analysis highlights several niche but important insights. First, keyword clustering and citation bursts reveal a growing interest in microbial metabolic biomarkers, underscoring their potential as next-generation diagnostic tools and therapeutic targets. This trend points to a shift towards investigating the microbiome–host metabolic axis, which remains an underexplored frontier in cancer biomarker research.
Second, in the PubMed database, we retrieved a total of 39 clinical trials related to metabolic biomarkers and cancer. Different metabolic biomarkers are currently being tested in a clinical trial environment. These indicators include but are not limited to leptin, adiponectin, insulin, glucose, lactic acid, short-chain fatty acids, glutamine, and MOTS-c, covering different functional categories such as energy metabolism and hormone regulation. Upon summarizing and analyzing these studies, we identified several emerging trends and research focuses in the current literature.Several studies have evaluated how structured lifestyle modifications influence metabolic biomarker profiles. For example, Aerobic and resistance exercise have been shown to improve inflammatory markers, enhance mitochondrial function, and promote overall metabolic health in cancer patients and survivors. In breast cancer survivors specifically, such exercise interventions significantly altered circulating levels of MOTS-c [53]. In addition, a clinical trial demonstrated that remotely delivered weight-loss interventions led to significant weight reduction in breast cancer survivors and positively influenced several metabolic biomarkers [54]. Other studies have focused on identifying metabolomic signatures for early detection and risk stratification. Salivary and urinary metabolomic profiling revealed small molecules—such as amino acid derivatives, bile acids, and carnitines—as potential non-invasive biomarkers for cancers including oral, bladder, and colorectal cancer [55–57]. These findings underscore the potential of metabolomic signatures as tools for diagnosis and recurrence surveillance. Beyond lifestyle interventions, pharmacological agents and nutraceuticals have also been explored for their impact on metabolic pathways. For instance, metformin was shown to remodel the tumor microenvironment in head and neck cancer [58], while polymorphisms in the vitamin D receptor were associated with lipid metabolism outcomes in breast cancer survivors [59]. In addition, we found that the success of clinical transformation often has several common characteristics : (1) clinical relevance : biomarkers such as insulin are associated with metabolic syndrome and cancer progression, and therefore have a high clinical relevance (2). Noninvasive sampling : Due to practicality, blood or urine, etc. are more likely to be tested and adopted (3). Cross-disease practicality : It has been evaluated in many diseases, including cancer, and is more likely to be successfully transformed (4). Prior regulatory approval : Biomarkers previously validated in other disease areas ( such as cardiovascular risk ) can be applied to tumor trials more quickly.
Despite the substantial increase in publications over the past decade, our findings reveal a notable lag in the clinical translation of metabolic biomarkers. Many identified biomarkers have not undergone rigorous validation or been integrated into clinical workflows. Issues related to analytical reproducibility and standardization continue to impede validation across different settings and populations. Additionally, the cost and technical complexity of advanced metabolomics assays can limit their feasibility in routine clinical practice. Third, many proposed metabolic biomarkers still lack prospective validation in large, diverse cohorts or integration with clinical decision-support tools, further delaying their adoption.
These niche findings offer actionable insights for researchers and policymakers aiming to prioritize funding and design future studies that address these critical challenges.
Limitations
The main source of data for this research is the Web of Science Core Collection (WoSCC) database, utilized to thoroughly investigate the comprehensive landscape, pinpoint research hotspots, and assess developmental trends within the discipline. This approach aids in deepening the understanding of the domain and provides substantial guidance for exploring future research directions. Nevertheless, it must be acknowledged that there are some significant limitations. To begin with, while the WoSCC database is celebrated for its exceptional quality and is often regarded as a premier tool and primary source for bibliometric analysis, depending exclusively on this database could lead to the exclusion of certain pertinent publications. Additionally, concentrating solely on publications in English may result in linguistic bias, limiting the relevance of the research outcomes and overlooking significant studies conducted in other languages. Nonetheless, despite these limitations, the outcomes derived from this study continue to be strong and provide significant insights and references for scholarly research in this area.
Conclusion
This research thoroughly discusses the primary areas of focus and the latest advancements in the realm of metabolic biomarkers associated with cancer. The main conclusions are outlined below:
The relationship between metabolic biomarkers and cancer has attracted considerable attention from researchers around the globe. Key players in this area include China, the United States, the United Kingdom, Japan, and Italy, and these countries have shown significant differences in the level of international cooperation, helping us understand how global partnerships affect the dissemination of knowledge and research development in this field.
In the field, the journals Cancers, Scientific Reports, and Metabolites are among the most productive. Remarkably, PLOS One is distinguished as the most frequently cited journal, underscoring its importance in this area.
Common cancers, including breast cancer, prostate cancer, colorectal cancer, and lung cancer, have become key focal points of research and are associated with the major trend of utilizing metabolic biomarkers for cancer treatment. These applications cover several important areas, including early disease diagnosis, disease differentiation, and treatment response prediction. While other types, such as gastric or pancreatic cancer, appear less frequently. This underscores potential underexplored areas for future investigation.
Researchers can advance the field of precision oncology through the use of artificial intelligence technologies, especially machine learning and deep learning, which allow for the identification of complex patterns within metabolomics data. The integration of multi-omics data with artificial intelligence significantly enhances the accuracy of early cancer diagnosis. However, challenges such as data integration, algorithm interpretability, and data security remain and require further exploration. While our study aimed to address questions such as the influence of multi-omics integration on biomarker discovery, our bibliometric data revealed relatively few explicit references to “multi-omics” approaches, suggesting that this integration remains an emerging but still limited trend in the field.
The metabolic axis between the microbiota and the host represents a complex interaction mediated by metabolites, which can regulate the host’s immunity and physiological functions. This interaction has a significant impact on the occurrence and progression of cancer. Future research should focus on elucidating its potential mechanisms and developing intervention strategies to improve the efficacy of cancer treatment.
Although the research on metabolic biomarkers continues to grow, clinical transformation is still lagging behind. Emerging directions include microbial markers, lifestyle interventions, and early metabolic diagnosis. Limited by insufficient verification, different standards and low clinical integration, the practical application is limited. In the future, priority should be given to supporting marker research with clinical relevance, non-invasive and cross-disease potential to accelerate the transformation process.
This study thoroughly investigates the trends and key areas regarding the use of metabolic biomarkers in cancer research, uncovering numerous valuable insights. The results offered not only equip researchers with a comprehensive background to thoroughly understand the main trajectories within this field but also establish a solid groundwork for pursuing new research paths. Our research offers clear guidance and a solid framework for future innovative inquiries by effectively identifying key advancements and potentially significant areas in current studies.
Supplementary Information
Author contributions
LZ contributed concepts and design; LZ and YS conducted data analysis and data interpretation and participated in the drafting of the manuscript. YM contributed to the revision of the manuscript. All authors have contributed. The article was reviewed and the submitted version was approved.
Funding
State Administration of Traditional Chinese Medicine High Level Key Discipline Construction Project of Traditional Chinese Medicine-Traditional Chinese Medicine External Treatment ( zyyzdxk-2023116 ).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and Trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. 10.1158/1055-9965.Epi-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: A review. Scand J Public Health. 2018;46(1):27–36. 10.1177/1403494817715400. [DOI] [PubMed] [Google Scholar]
- 3.Sedeta E, Sung H, Laversanne M, Bray F, Jemal A. Recent mortality patterns and time trends for the major cancers in 47 countries worldwide. Cancer Epidemiol Biomarkers Prev. 2023;32(7):894–905. 10.1158/1055-9965.Epi-22-1133. [DOI] [PubMed] [Google Scholar]
- 4.Lortet-Tieulent J, Georges D, Bray F, Vaccarella S. Profiling global cancer incidence and mortality by socioeconomic development. Int J Cancer. 2020;147(11):3029–36. 10.1002/ijc.33114. [DOI] [PubMed] [Google Scholar]
- 5.Ni Y, Xie G, Jia W. Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. J Proteome Res. 2014;13(9):3857–70. 10.1021/pr500443c. [DOI] [PubMed] [Google Scholar]
- 6.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: A review. Clin Cancer Res. 2009;15(2):431–40. 10.1158/1078-0432.Ccr-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J, Batra S, Zhang J. Asparagine: A metabolite to be targeted in cancers. Metabolites. 2021. 10.3390/metabo11060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11(12):835–48. 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen S, Kawahara B, Mahata SK, Tsai R, Yoon A, Hwang L, et al. Cystathionine: A novel oncometabolite in human breast cancer. Arch Biochem Biophys. 2016;604:95–102. 10.1016/j.abb.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014;12:164. 10.1186/1477-7819-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bamji-Stocke S, van Berkel V, Miller DM, Frieboes HB. A review of Metabolism-Associated biomarkers in lung cancer diagnosis and treatment. Metabolomics. 2018;14(6):81. 10.1007/s11306-018-1376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Mahendran R, Tham SM, Thamboo TP, Chionh BJ, Lim YX, et al. Tryptophan-Kynurenine ratio as a biomarker of bladder cancer. BJU Int. 2021;127(4):445–53. 10.1111/bju.15205. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Tian SL, Jin D, Liu B, Wang W, Chang H, et al. The role of bile acid subtypes in the diagnosis of cholangiocarcinoma. Asia Pac J Clin Oncol. 2022;18(2):e163–72. 10.1111/ajco.13588. [DOI] [PubMed] [Google Scholar]
- 14.Croteau E, Renaud JM, Richard MA, Ruddy TD, Bénard F, deKemp RA. Pet metabolic biomarkers for cancer. Biomark Cancer. 2016;8(Suppl 2):61–9. 10.4137/bic.S27483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsoli M, Daskalakis K, Kassi E, Kaltsas G, Tsolakis AV. A critical appraisal of contemporary and novel biomarkers in pheochromocytomas and adrenocortical tumors. Biology (Basel). 2021. 10.3390/biology10070580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Xiao X, Sadeghi F, Feychting M, Hammar N, Fang F, et al. Blood metabolic biomarkers and the risk of head and neck cancer: an epidemiological study in the Swedish amoris cohort. Cancer Lett. 2023;557:216091. 10.1016/j.canlet.2023.216091. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Tang Q, Zhang K, Huang Q, Ding Y, Jin B, et al. Altered expression of the L-Arginine/Nitric oxide pathway in ovarian cancer: metabolic biomarkers and biological implications. BMC Cancer. 2023;23(1):844. 10.1186/s12885-023-11192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-Related mortality. Physiol Rev. 2015;95(3):727–48. 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Q, Zhan C, Shen Y, Xu Y, Ren B, Feng Z, et al. Blood lipid metabolic biomarkers are emerging as significant prognostic indicators for survival in cancer patients. BMC Cancer. 2024;24(1):1549. 10.1186/s12885-024-13265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Yang W, Liu L, Zhou S, Jin X, Zhao M, et al. Publication trends and hotspots for cervical cancer screening biomarker: A bibliometric analysis. Discov Oncol. 2025;16(1):1151. 10.1007/s12672-025-02936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao Y, Xie D, Li Z, Cao S, Zhao D. Global research trends on biomarkers for cancer immunotherapy: visualization and bibliometric analysis. Hum Vaccin Immunother. 2025;21(1):2435598. 10.1080/21645515.2024.2435598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Eck NJ, Waltman L. Software survey: Vosviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C. Citespace ii: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inform Sci Technol. 2005;57(3):359–77. 10.1002/asi.20317. [Google Scholar]
- 24.Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct Stage-Specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968–76. 10.1038/s41591-019-0458-7. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20(3):303–e145. 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer. 2021;7(7):647–60. 10.1016/j.trecan.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Xiong W, Liang Z, Wang J, Zeng Z, Kołat D, et al. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J Hematol Oncol. 2024;17(1):33. 10.1186/s13045-024-01541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Xie M, Lau HC, Zeng R, Zhang R, Wang L, et al. Effects of gut microbiota on immune checkpoint inhibitors in Multi-Cancer and as microbial biomarkers for predicting therapeutic response. Med. 2025;6(3):100530. 10.1016/j.medj.2024.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Park YH, Shin HW, Jung AR, Kwon OS, Choi YJ, Park J, et al. Prostate-Specific extracellular vesicles as a novel biomarker in human prostate cancer. Sci Rep. 2016;6:30386. 10.1038/srep30386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhagirath D, Liston M, Akoto T, Lui B, Bensing BA, Sharma A, et al. Novel, Non-Invasive markers for detecting therapy induced neuroendocrine differentiation in Castration-Resistant prostate cancer patients. Sci Rep. 2021;11(1):8279. 10.1038/s41598-021-87441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamed MA, Wasinger V, Wang Q, Graham P, Malouf D, Bucci J, et al. Prostate Cancer-Derived extracellular vesicles metabolic biomarkers: emerging roles for diagnosis and prognosis. J Control Release. 2024;371:126–45. 10.1016/j.jconrel.2024.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, Yang J, Chen Y, Lin J, Huang W, Li M. Association of Circulating metabolic biomarkers with risk of lung cancer: A Population-Based prospective cohort study. BMC Med. 2025;23(1):176. 10.1186/s12916-025-03993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong S, Wang Q, Huang J, Huang R, Chen S, Cheng X, et al. Lc-Ms/Ms Platform-Based serum untargeted screening reveals the diagnostic biomarker panel and molecular mechanism of breast cancer. Methods. 2024;222:100–11. 10.1016/j.ymeth.2024.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Jasbi P, Shi X, Turner C, Hrovat J, Liu L, et al. Early breast cancer detection using untargeted and targeted metabolomics. J Proteome Res. 2021;20(6):3124–33. 10.1021/acs.jproteome.1c00019. [DOI] [PubMed] [Google Scholar]
- 35.His M, Viallon V, Dossus L, Gicquiau A, Achaintre D, Scalbert A, et al. Prospective analysis of Circulating metabolites and breast cancer in epic. BMC Med. 2019;17(1):178. 10.1186/s12916-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lécuyer L, Victor Bala A, Deschasaux M, Bouchemal N, Nawfal Triba M, Vasson MP, et al. Nmr metabolomic signatures reveal predictive plasma metabolites associated with Long-Term risk of developing breast cancer. Int J Epidemiol. 2018;47(2):484–94. 10.1093/ije/dyx271. [DOI] [PubMed] [Google Scholar]
- 37.Suman S, Sharma RK, Kumar V, Sinha N, Shukla Y. Metabolic fingerprinting in breast cancer stages through (1)H Nmr Spectroscopy-Based metabolomic analysis of plasma. J Pharm Biomed Anal. 2018;160:38–45. 10.1016/j.jpba.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Avuthu N, Guda C. Meta-Analysis of altered gut microbiota reveals microbial and metabolic biomarkers for colorectal cancer. Microbiol Spectr. 2022;10(4):e0001322. 10.1128/spectrum.00013-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heo YJ, Hwa C, Lee GH, Park JM, An JY. Integrative Multi-Omics approaches in cancer research: from biological networks to clinical subtypes. Mol Cells. 2021;44(7):433–43. 10.14348/molcells.2021.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández-Lemus E, Ochoa S. Methods for Multi-Omic data integration in cancer research. Front Genet. 2024;15:1425456. 10.3389/fgene.2024.1425456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song H, Tang X, Liu M, Wang G, Yuan Y, Pang R, et al. Multi-Omic analysis identifies metabolic biomarkers for the early detection of breast cancer and therapeutic response prediction. iScience. 2024;27(9):110682. 10.1016/j.isci.2024.110682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong S, Huang R, Wang M, Lian F, Wang Q, Liao Z, et al. Comprehensive analysis of the metabolomics and transcriptomics uncovers the dysregulated network and potential biomarkers of triple negative breast cancer. J Transl Med. 2024;22(1):1016. 10.1186/s12967-024-05843-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anh NK, Lee A, Phat NK, Yen NTH, Thu NQ, Tien NTN, et al. Combining metabolomics and machine learning to discover biomarkers for Early-Stage breast cancer diagnosis. PLoS ONE. 2024;19(10):e0311810. 10.1371/journal.pone.0311810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuwabara H, Katsumata K, Iwabuchi A, Udo R, Tago T, Kasahara K, et al. Salivary metabolomics with machine learning for colorectal cancer detection. Cancer Sci. 2022;113(9):3234–43. 10.1111/cas.15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21(2):141–62. 10.1038/s41573-021-00339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, et al. Metaboanalyst 5.0: narrowing the gap between Raw spectra and functional insights. Nucleic Acids Res. 2021;49(W1):W388–96. 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The Microbiome and human cancer. Science. 2021;371(6536). 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed]
- 48.Yin T, Zhang X, Xiong Y, Li B, Guo D, Sha Z, et al. Exploring gut microbial metabolites as key players in Inhibition of cancer progression: mechanisms and therapeutic implications. Microbiol Res. 2024;288:127871. 10.1016/j.micres.2024.127871. [DOI] [PubMed] [Google Scholar]
- 49.He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic Cd8(+) T cell immunity. Cell Metab. 2021;33(5):988–e10007. 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Kim M, Friesen L, Park J, Kim HM, Kim CH. Microbial Metabolites, Short-Chain fatty Acids, restrain tissue bacterial Load, chronic Inflammation, and associated cancer in the colon of mice. Eur J Immunol. 2018;48(7):1235–47. 10.1002/eji.201747122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu K, Jia N, Shi H, Ran Y. Current and future research on the association between gut microbiota and breast cancer. Front Microbiol. 2023;14:1272275. 10.3389/fmicb.2023.1272275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng Y, Gu J, Liu F, Wang P, Wang X, Si C, et al. Integrated analysis of microbiota and gut microbial metabolites in blood for breast cancer. mSystems. 2024;9(11):e0064324. 10.1128/msystems.00643-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dieli-Conwright CM, Sami N, Norris MK, Wan J, Kumagai H, Kim SJ, et al. Effect of aerobic and resistance exercise on the mitochondrial peptide Mots-C in Hispanic and Non-Hispanic white breast cancer survivors. Sci Rep. 2021;11(1):16916. 10.1038/s41598-021-96419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santa-Maria CA, Coughlin JW, Sharma D, Armanios M, Blackford AL, Schreyer C, et al. The effects of a Remote-Based weight loss program on Adipocytokines, metabolic Markers, and telomere length in breast cancer survivors: the Power-Remote trial. Clin Cancer Res. 2020;26(12):3024–34. 10.1158/1078-0432.Ccr-19-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishikawa S, Sugimoto M, Kitabatake K, Sugano A, Nakamura M, Kaneko M, et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci Rep. 2016;6:31520. 10.1038/srep31520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loras A, Trassierra M, Sanjuan-Herráez D, Martínez-Bisbal MC, Castell JV, Quintás G, et al. Bladder cancer recurrence surveillance by urine metabolomics analysis. Sci Rep. 2018;8(1):9172. 10.1038/s41598-018-27538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C, Zhou S, Chang H, Zhuang F, Shi Y, Chang L, et al. Metabolomic profiling identified serum metabolite biomarkers and related metabolic pathways of colorectal cancer. Dis Markers. 2021;2021:6858809. 10.1155/2021/6858809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curry J, Johnson J, Tassone P, Vidal MD, Menezes DW, Sprandio J, et al. Metformin effects on head and neck squamous carcinoma microenvironment: window of opportunity trial. Laryngoscope. 2017;127(8):1808–15. 10.1002/lary.26489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kazemian E, Amouzegar A, Akbari ME, Moradi N, Gharibzadeh S, Jamshidi-Naeini Y, et al. Vitamin D receptor gene polymorphisms affecting changes in visceral Fat, waist circumference and lipid profile in breast cancer survivors supplemented with vitamin D3. Lipids Health Dis. 2019;18(1):161. 10.1186/s12944-019-1100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.