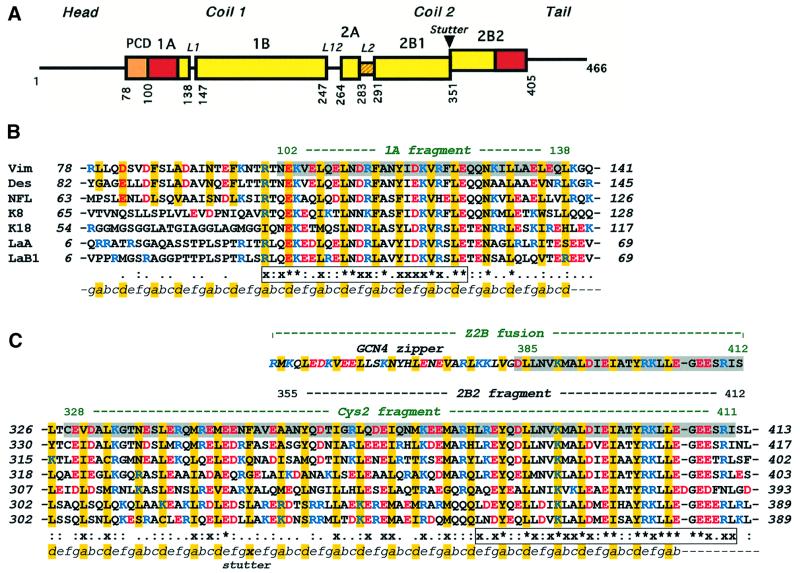
Fig. 1. (A) Primary structure of IF proteins. Schematic diagram of human vimentin. Rectangles show α-helical segments, including the pre-coil domain (PCD). (B) Sequence alignment of the 1A segments of human IF proteins including vimentin, desmin, neurofilament L protein, cytokeratins 8 and 18, and nuclear lamins A and B1. (C) Similar alignment of the 2B segments. Vimentin fragments 1A, Cys2, Z2B and 2B2 are highlighted. The heptad repeats are marked as abcdefg, with core positions highlighted with yellow. Basic and acidic residues are shown in blue and red, respectively. The line below the alignment shows the sequence similarity score s of a particular residue in the seven proteins: ‘*’, s = 1.0 (absolutely conserved); ‘x’, 0.75≤s<1.0; ‘:’, 0.5≤s<0.75; ‘.’, 0.25≤s<0.5 (see Materials and methods for details). The two most conserved regions within the 1A segment and in the C-terminal part of the 2B segment, respectively, are shown in boxes.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.