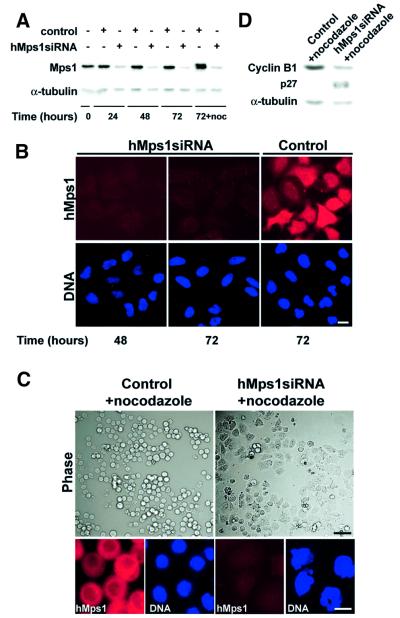
Fig. 5. Silencing of hMps1 by siRNA duplex abolishes the spindle assembly checkpoint. (A) Western blotting shows effective silencing of hMps1 in HeLa S3 cells (transfection efficiency = 90%). Following mock transfection or transfection with hMps1 siRNA duplex, cell extracts were prepared at the time points indicated. Equal amounts of protein were separated by SDS–PAGE and probed by western blotting with anti-hMps1 mAb N1 (upper panel) and anti-α-tubulin antibody as a loading control (lower panel). In the sample marked noc, nocodazole (50 ng/ml) was added at t = 48 h for an additional 24 h. (B) Immunofluorescence microscopy shows effective silencing of hMps1 in HeLaS3 cells. Cells were transfected as described in (A), fixed and permeabilized with paraformaldehyde/Triton X-100, and stained for hMps1 (upper panels) and DNA (lower panels) using anti-hMps1 mAb N1 and DAPI, respectively. Bar = 10 µm. (C) Analysis of HeLa S3 cells after mock transfection (left) or hMps1 silencing by siRNA duplex (right) and subsequent exposure to spindle damage. Nocodazole (50 ng/ml) was added 48 h post-transfection for 24 h before cells were analyzed. Upper panels: phase-contrast pictures. Lower panels: cells stained for hMps1 (left) and DNA (right). Bars = 1 µm (upper panels) and 10 µm (lower panels). (D) Cell extracts, prepared from the samples described in (C), were probed by western blotting with anti-cyclin B1 and anti-p27Kip1 antibodies, respectively. Anti-α-tubulin was used as a loading control.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.