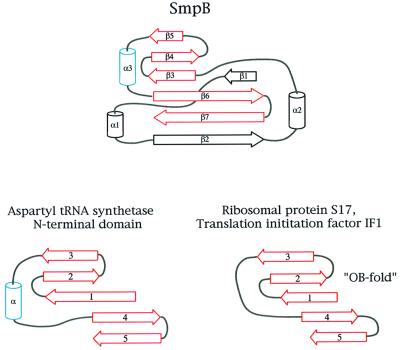
Fig. 9. Relationship of SmpB to other RNA-binding proteins. The structure of SmpB contains an embedded oligonucleotide binding (OB-fold); in this respect, SmpB is related to several other proteins associated with the translational apparatus. Ribosomal protein S17 and translation initiation factor IF1 are other members of the OB-fold family of proteins, and contain a five-stranded antiparallel β-sheet (shown in red), with the strands connected in a Greek-key topology. The N-terminal domain of aspartyl tRNA synthetase also contains the OB-fold within its structure, as does SmpB. The secondary structures of the aspartyl tRNA synthetase, S17, IF1 and SmpB are derived from PDB entries 1ASZ, 1RIP, 1AH9 and 1K8H, respectively.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.