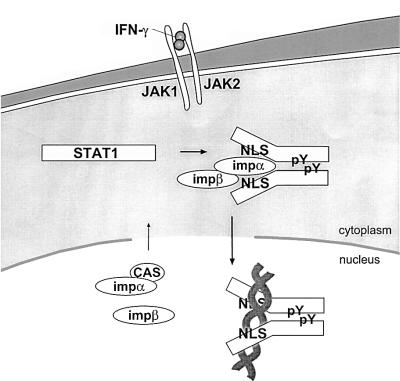
Fig. 8. Conceptual model of STAT1 nuclear translocation. Latent STAT1 exists in the cytoplasm as a monomer. Following IFN-γ stimulation, STAT1 is tyrosine phosphorylated and dimerizes via intermolecular phosphotyrosine (pY)-SH2 domains. Dimerization results in a conformational change that allows the STAT1 NLS on each monomer to become functional. The importin-α5 (impα) shuttling receptor recognizes and binds to the NLS on the STAT1 dimer and effects translocation into the nucleus in association with importin-β (impβ). In the nucleus, STAT1 recognition of a specific DNA target leads to dissociation of importin-α5. Importin-α5 can be recycled to the cytoplasm by the cellular apoptosis susceptibility protein (CAS) export receptor.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.