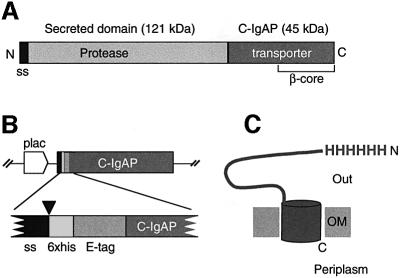
Fig. 1. Structural organization of the IgA protease and HEβ hybrid. (A) Schematic representation of the IgA protease precursor (∼170 kDa) from N.gonorroheae showing the position of the N-terminal signal sequence (ss), the secreted protease module (∼120 kDa) and the transporter C-domain (∼45 kDa; C-IgAP). The ∼30 kDa β-core region within C-IgAP is also labeled. (B) Organization of the relevant insert of plasmid pHEβ encoding the HEβ hybrid under the control of the IPTG-inducible lac promoter (plac). The heb gene chimera is an in-frame fusion of DNA segments encoding the pelB N-terminal signal sequence (ss), polyhistidine (6×his) and E-tag peptides, and the C-IgAP transporter domain. The site of cleavage of the signal peptidase (just before the His6 peptide) is indicated by an arrowhead. (C) Representation of topology of HEβ in the OM showing the His6 N-passenger domain exposed to the extracellular space (Out) and the C-terminus toward the periplasm. The putative β-barrel of HEβ is depicted as a cylinder embedded in the OM.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.