Abstract
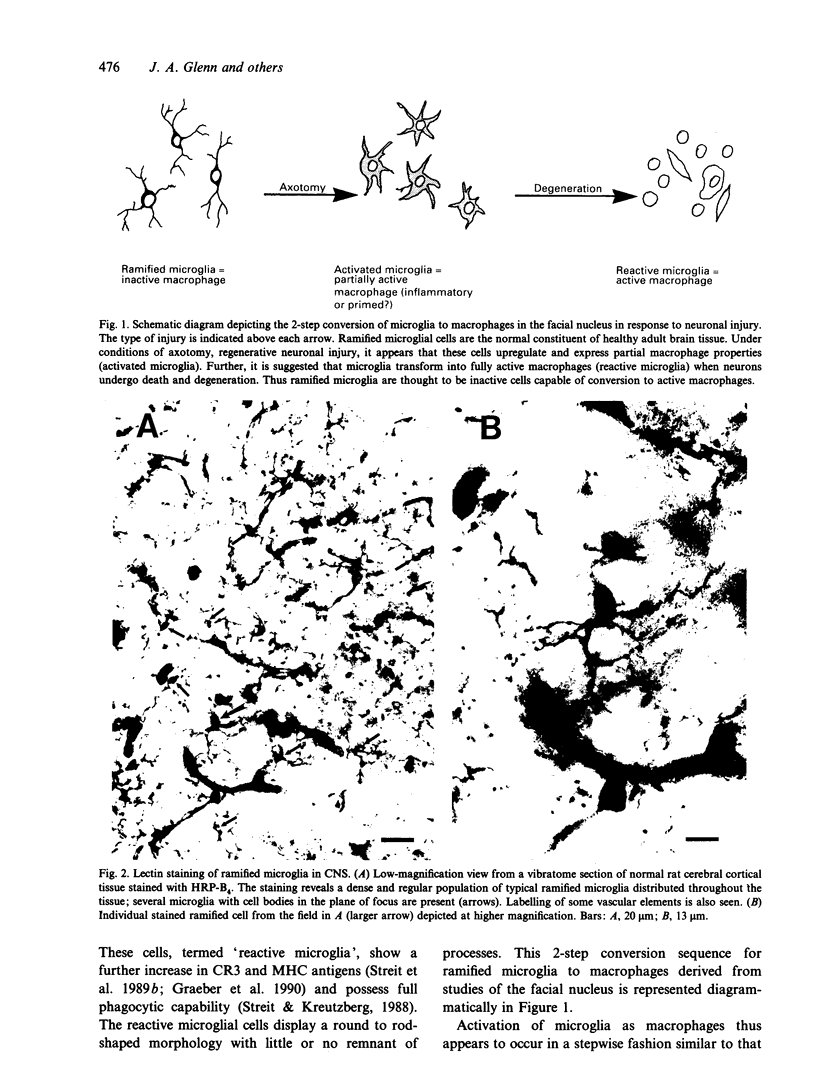
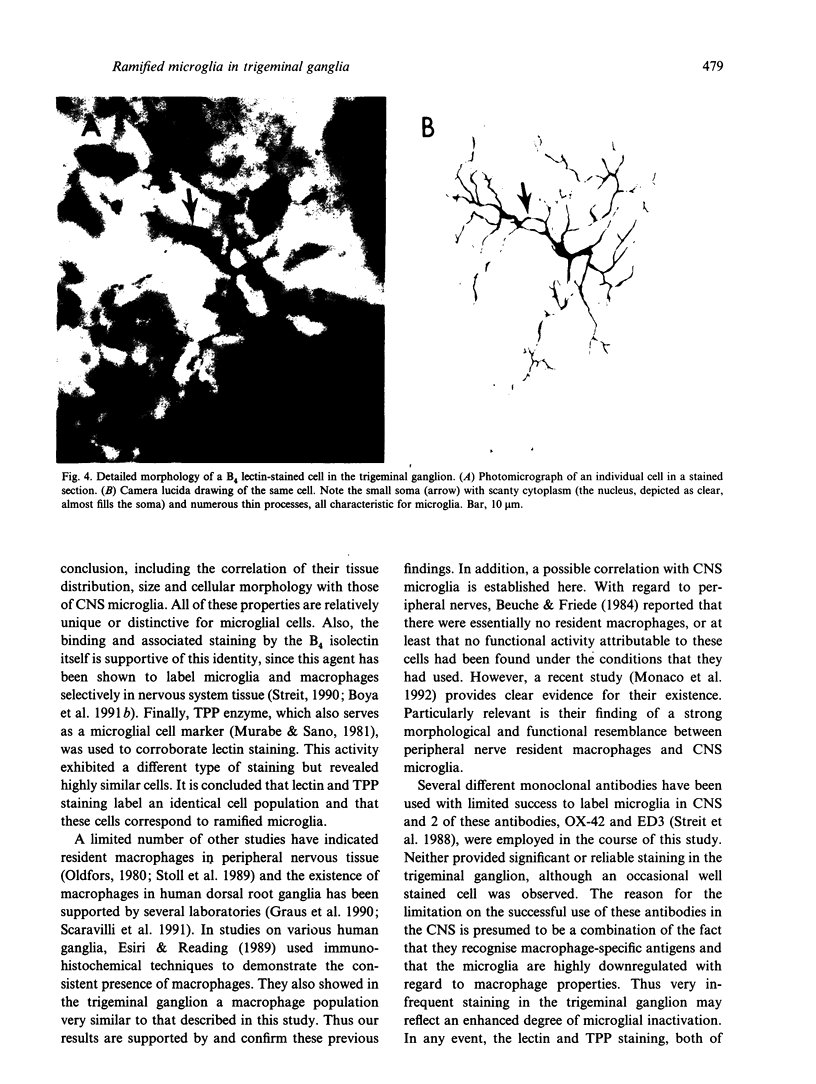
Of the 4 major cell types in CNS parenchyma, microglia appear to serve the unique functional role of tissue macrophages. The distribution of equivalent cells in the PNS is unclear. Recently, the B4 isolectin of Griffonia simplicifolia was shown to bind selectively to microglia as well as to other macrophages under specific conditions. In the present study, this lectin was used to assess the existence of macrophages in the rat trigeminal ganglion. Vibratome sections of fixed ganglia were incubated with horseradish peroxidase (HRP)-conjugated isolectin, an HRP reaction subsequently performed, and sections processed for histology and viewed by light microscopy. Staining activity was found to be localised to a population of cells throughout the ganglion. These cells possessed small oval somata and several thin crenated processes, an appearance typical of ramified microglia. Stained cells also exhibited a regular, evenly spaced tissue distribution similar to CNS microglia. Finally, similar cells were also labelled by thiamine pyrophosphatase histochemistry, a cellular marker for CNS microglia/macrophages. It was concluded that there are microglia-like macrophages in the trigeminal ganglion and that these cells may function in immune reactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Ambalavanar R., Morris R. The distribution of binding by isolectin I-B4 from Griffonia simplicifolia in the trigeminal ganglion and brainstem trigeminal nuclei in the rat. Neuroscience. 1992;47(2):421–429. doi: 10.1016/0306-4522(92)90256-2. [DOI] [PubMed] [Google Scholar]
- Beuche W., Friede R. L. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984 Oct;13(5):767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Boya J., Calvo J. L., Carbonell A. L., Borregon A. A lectin histochemistry study on the development of rat microglial cells. J Anat. 1991 Apr;175:229–236. [PMC free article] [PubMed] [Google Scholar]
- Boya J., Carbonell A. L., Calvo J. L., Borregon A. Microglial cells in the central nervous system of the rabbit and rat: cytochemical identification using two different lectins. Acta Anat (Basel) 1991;140(3):250–253. doi: 10.1159/000147064. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Reading M. C. Macrophages, lymphocytes and major histocompatibility complex (HLA) class II antigens in adult human sensory and sympathetic ganglia. J Neuroimmunol. 1989 Aug;23(3):187–193. doi: 10.1016/0165-5728(89)90050-7. [DOI] [PubMed] [Google Scholar]
- Glenn J. A., Jordan F. L., Thomas W. E. Further studies on the identification of microglia in mixed brain cell cultures. Brain Res Bull. 1989 Jun;22(6):1049–1052. doi: 10.1016/0361-9230(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Glenn J. A., Ward S. A., Stone C. R., Booth P. L., Thomas W. E. Characterisation of ramified microglial cells: detailed morphology, morphological plasticity and proliferative capability. J Anat. 1992 Feb;180(Pt 1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kiefer R., Schoen S. W., Kreutzberg G. W. New expression of myelomonocytic antigens by microglia and perivascular cells following lethal motor neuron injury. J Neuroimmunol. 1990 May;27(2-3):121–132. doi: 10.1016/0165-5728(90)90061-q. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kreutzberg G. W. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1988 Sep;21(1):18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Tetzlaff W., Streit W. J., Kreutzberg G. W. Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett. 1988 Mar 10;85(3):317–321. doi: 10.1016/0304-3940(88)90585-x. [DOI] [PubMed] [Google Scholar]
- Graus F., Campo E., Cruz-Sanchez F., Ribalta T., Palacin A. Expression of lymphocyte, macrophage and class I and II major histocompatibility complex antigens in normal human dorsal root ganglia. J Neurol Sci. 1990 Sep;98(2-3):203–211. doi: 10.1016/0022-510x(90)90261-k. [DOI] [PubMed] [Google Scholar]
- Jordan F. L., Thomas W. E. Brain macrophages: questions of origin and interrelationship. Brain Res. 1988 Apr-Jun;472(2):165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- Monaco S., Gehrmann J., Raivich G., Kreutzberg G. W. MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J Neurocytol. 1992 Sep;21(9):623–634. doi: 10.1007/BF01191724. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Thiaminepyrophosphatase activity in the plasma membrane of microglia. Histochemistry. 1981;71(1):45–52. doi: 10.1007/BF00592569. [DOI] [PubMed] [Google Scholar]
- Oldfors A. Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol. 1980;49(1):43–49. doi: 10.1007/BF00692218. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988 Jun;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Scaravilli F., Giometto B., Chimelli L., Sinclair E. Macrophages in human sensory ganglia: an immunohistochemical and ultrastructural study. J Neurocytol. 1991 Jul;20(7):609–624. doi: 10.1007/BF01215268. [DOI] [PubMed] [Google Scholar]
- Stoll G., Griffin J. W., Li C. Y., Trapp B. D. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989 Oct;18(5):671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- Streit W. J. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4). J Histochem Cytochem. 1990 Nov;38(11):1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989 Aug;105(2):115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Peripheral nerve lesion produces increased levels of major histocompatibility complex antigens in the central nervous system. J Neuroimmunol. 1989 Feb;21(2-3):117–123. doi: 10.1016/0165-5728(89)90167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Response of endogenous glial cells to motor neuron degeneration induced by toxic ricin. J Comp Neurol. 1988 Feb 8;268(2):248–263. doi: 10.1002/cne.902680209. [DOI] [PubMed] [Google Scholar]
- Thomas W. E. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992 Jan-Apr;17(1):61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]