Abstract
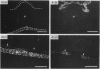
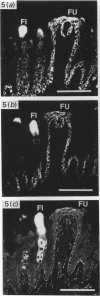
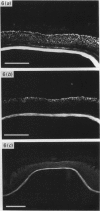
Four monoclonal antibodies raised against murine embryonic palatal epithelium were used to stain frontal cryosections of embryonic mouse heads (d 11-15). One antibody (6A11) recognised the nasal epithelium at the angle of the palate prior to shelf elevation (embryonic d 11-d 13) and the epithelia at the tips of the approximated shelves (embryonic d 14.5). Two further antibodies (2H6 and 3H2) recognised epitopes on the entire palatal epithelium until shelf elevation (embryonic d 14.5) when their expression ceased. The epitope recognised by antibody 6D3 was absent from the palatal epithelium until embryonic d 13 when it displayed region specific expression, being present on the nasal aspect in the midpalate and the tip of the palate posteriorly. On embryonic d 14.5 staining of the medial edge epithelia was present in all regions. Whilst the nasal palatal epithelium stained in the anterior and mid regions, only patchy staining of the oral epithelium was detected. By embryonic d 15 staining was present throughout the palatal epithelia. These antibodies are directed against intracellular epitopes, possibly keratins. Indeed, immunoblotting assays revealed that antibody 2H6 recognised keratin 18. The developmental regulation of the epitopes recognised by the antibodies corresponds with differentiative events occurring during murine palatogenesis.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Leube R. E., Achtstätter T., Moll R., Franke W. W. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J Cell Biol. 1988 May;106(5):1635–1648. doi: 10.1083/jcb.106.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley L. L., Morris-Wiman J. Computer-assisted analysis of hyaluronate distribution during morphogenesis of the mouse secondary palate. Development. 1987 Aug;100(4):629–635. doi: 10.1242/dev.100.4.629. [DOI] [PubMed] [Google Scholar]
- Carette M. J., Ferguson M. W. The fate of medial edge epithelial cells during palatal fusion in vitro: an analysis by DiI labelling and confocal microscopy. Development. 1992 Feb;114(2):379–388. doi: 10.1242/dev.114.2.379. [DOI] [PubMed] [Google Scholar]
- Carette M. J., Lane E. B., Ferguson M. W. Differentiation of mouse embryonic palatal epithelium in culture: selective cytokeratin expression distinguishes between oral, medial edge and nasal epithelial cells. Differentiation. 1991 Aug;47(3):149–161. doi: 10.1111/j.1432-0436.1991.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Ferguson M. W. Palate development: mechanisms and malformations. Ir J Med Sci. 1987 Nov;156(11):309–315. doi: 10.1007/BF02951261. [DOI] [PubMed] [Google Scholar]
- Ferguson W. J. Epithelial-mesenchymal interactions during vertebrate palatogenesis. Curr Top Dev Biol. 1984;19:137–164. doi: 10.1016/s0070-2153(08)60398-1. [DOI] [PubMed] [Google Scholar]
- Fitchett J. E., Hay E. D. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989 Feb;131(2):455–474. doi: 10.1016/s0012-1606(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Greene R. M., Kochhar D. M. Surface coat on the epithelium of developing palatine shelves in the mouse as revealed by electron microscopy. J Embryol Exp Morphol. 1974 Jun;31(3):683–692. [PubMed] [Google Scholar]
- Greene R. M., Pratt R. M. Correlation between cyclic-AMP levels and cytochemical localization of adenylate cyclase during development of the secondary palate. J Histochem Cytochem. 1979 May;27(5):924–931. doi: 10.1177/27.5.225376. [DOI] [PubMed] [Google Scholar]
- Greene R. M., Pratt R. M. Developmental aspects of secondary palate formation. J Embryol Exp Morphol. 1976 Oct;36(2):225–245. [PubMed] [Google Scholar]
- Hudson C. D., Shapiro B. L. A radioautographic study of deoxyribonucleic acid synthesis in embryonic rat palatal shelf epithelium with reference to the concept of programmed cell death. Arch Oral Biol. 1973 Jan;18(1):77–84. doi: 10.1016/0003-9969(73)90022-8. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Lehto V. P., Virtanen I. Keratin filaments of mouse epithelial cells are rapidly affected by epidermal growth factor. J Cell Biol. 1981 Aug;90(2):537–541. doi: 10.1083/jcb.90.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Bártek J., Purkis P. E., Leigh I. M. Keratin antigens in differentiating skin. Ann N Y Acad Sci. 1985;455:241–258. doi: 10.1111/j.1749-6632.1985.tb50415.x. [DOI] [PubMed] [Google Scholar]
- Luke D. A. Epithelial proliferation and development of rugae in relation to palatal shelf elevation in the mouse. J Anat. 1984 Mar;138(Pt 2):251–258. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Morgan P. R., Pratt R. M. Ultrastructure of the expected fusion zone in rat fetuses with diazo-oxo-norleucine (DON)-induced cleft palate. Teratology. 1977 Jun;15(3):281–289. doi: 10.1002/tera.1420150310. [DOI] [PubMed] [Google Scholar]
- Ouellet T., Lussier M., Bélanger C., Kessous A., Royal A. Differential expression of keratin genes during mouse development. Dev Biol. 1986 Feb;113(2):282–287. doi: 10.1016/0012-1606(86)90163-6. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Hassell J. R. Appearance and distribution of carbohydrate-rich macromolecules on the epithelial surface of the developing rat palatal shelf. Dev Biol. 1975 Jul;45(1):192–198. doi: 10.1016/0012-1606(75)90253-5. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Martin G. R. Epithelial cell death and cyclic AMP increase during palatal development. Proc Natl Acad Sci U S A. 1975 Mar;72(3):874–877. doi: 10.1073/pnas.72.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Moesker O., Huysmans A., Schaart G., Westerhof G., Wagenaar S. S., Herman C. J., Vooijs G. P. Intermediate filament proteins in the study of tumor heterogeneity: an in-depth study of tumors of the urinary and respiratory tracts. Ann N Y Acad Sci. 1985;455:614–634. doi: 10.1111/j.1749-6632.1985.tb50440.x. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Winter H., Hill M. W., Mackenzie I. C. The keratin polypeptide patterns in heterotypically recombined epithelia of skin and mucosa of adult mouse. Differentiation. 1984;26(2):144–153. doi: 10.1111/j.1432-0436.1984.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Winter H. Keratin polypeptide analysis in fetal and in terminally differentiating newborn mouse epidermis. Differentiation. 1982;22(1):19–24. doi: 10.1111/j.1432-0436.1982.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Schüpbach P. M., Chamberlain J. G., Schroeder H. E. Development of the secondary palate in the rat: a scanning electron microscopic study. J Craniofac Genet Dev Biol. 1983;3(2):159–177. [PubMed] [Google Scholar]
- Sharpe P. M., Ferguson M. W. Mesenchymal influences on epithelial differentiation in developing systems. J Cell Sci Suppl. 1988;10:195–230. doi: 10.1242/jcs.1988.supplement_10.15. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman R. E., Ross L. M., Meller S. M. Alterations in the epithelial surface of A-Jax mouse palatal shelves prior to and during palatal fusion: a scanning electron microscopic study. Anat Rec. 1973 Jul;176(3):361–375. doi: 10.1002/ar.1091760311. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Fujimoto T. The anterior half of mouse palatal shelf is elevated by a remodeling movement. Acta Anat (Basel) 1986;125(1):37–41. doi: 10.1159/000146134. [DOI] [PubMed] [Google Scholar]