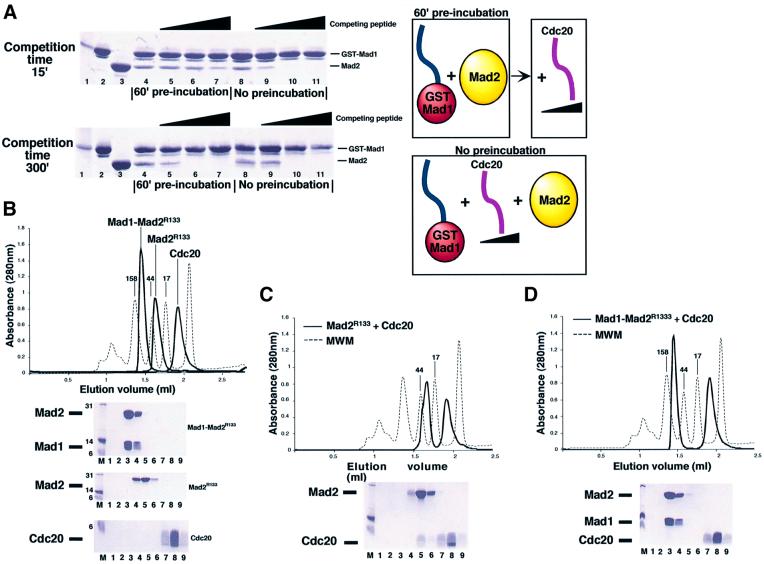
Fig. 5. Analysis of the postulated exchange mechanism. (A) Increasing concentrations of a Cdc20 synthetic peptide were incubated with Mad2, which had or had not been previously incubated with solid phase-bound GST–Mad1523–550. In the pre-incubation experiment the complex is allowed to form for 60 min, and the competing peptide is added afterwards. If no pre-incubation is allowed, the three components are mixed at the same time and incubated for 15 or 300 min. A diagrammatic representation is shown on the right-hand side. After incubation, beads and bound proteins are collected by centrifugation, washed and analysed by SDS–PAGE. Molecular weight marker, input GST–Mad1523–550 and Mad2 are shown on lanes 1–3. (B) Superimposed SEC profiles of isolated Mad1–Mad2, Mad2R133A and Cdc20111–150 synthetic peptide. The proteins/peptides run as single peaks by SEC. SDS–PAGE separation of collected fractions are also shown. Nine 100 µl fractions (1–9) were collected from 1.2 to 2.1 ml. (C) When Mad2R133A is incubated with a 5-fold excess of Cdc20111–150 and separated by SEC, a Mad2–Cdc20 complex forms [compare lanes 4–5 in (B) and (C)]. (D) When the Mad1–Mad2 complex is incubated with Cdc20111–150, no binding is observed. Furthermore, the peptide is unable to disrupt the complex, as no Mad2–Cdc20 complex or free Mad1 is detected.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.