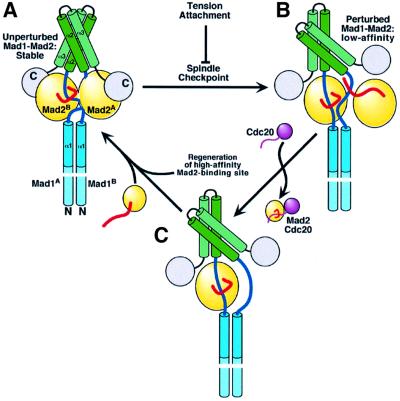
Fig. 6. Model for Mad1–Mad2 function in the spindle checkpoint. (A) Unperturbed Mad1–Mad2 will not exchange Mad2 with Cdc20. Our structure of Mad1–Mad2 may be related to this non-exchanging, high-affinity Mad1–Mad2 complex. (B) Perturbation by the spindle checkpoint decreases Mad2 affinity for Mad1. A monovalent Mad1–Mad2 interaction is low affinity, suggesting that decreased affinity may ensue if the Mad1–Mad2 heads become independent as an effect of reciprocal rotation. Cdc20 now competes with Mad1 for Mad2 binding. How Mad2 is re-loaded on to the complex is unclear, but it is reasonable to think that a new Mad2 binding site is generated to capture Mad2 with high affinity. (C) This high-affinity site would restore the unperturbed complex, and this cycle may continue until the attachment and/or tension dampen the checkpoint signal, preventing further release of Mad2 on to Cdc20.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.