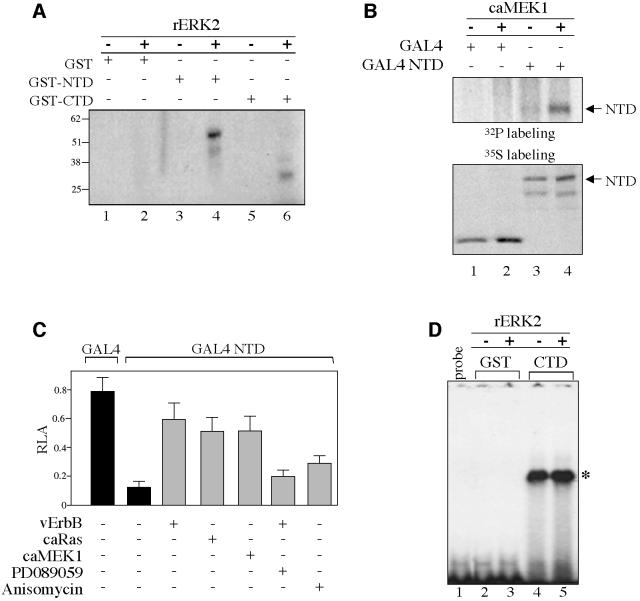
Fig. 3. EGF signaling inhibits TIEG2 N-terminal repression activity. (A) Equal amounts of GST fusion proteins carrying the N-terminal (GST–NTD) or the C-terminal (GST–CTD) domains of TIEG2 or GST alone were incubated with recombinant ERK2 (rERK2) (20 ng/µl) and [γ-32P]ATP as described in Materials and methods. Phosphorylation of the GST proteins was visualized by autoradiography. Note that rERK2 strongly phosphorylates GST–NTD (lane 3 versus 4) and to a lesser degree GST–CTD (lane 5 versus 6). GST alone is not phosphorylated (lane 1 versus 2). (B) CHO cells were transfected with TIEG2 NTD expressed as a GAL4 DBD fusion protein (GAL4 NTD) or GAL4 DBD alone (GAL4), with or without caMEK1. At 24 h post-transfection, cells were metabolically labeled with [32P]orthophosphate and subjected to anti-GAL4 immunoprecipitation. Phosphorylation of TIEG2 NTD was detected by autoradiography. Note that caMEK1 expression leads to increased phosphorylation of TIEG2 NTD (lane 3 versus 4). GAL4 DBD alone is not phosphorylated (lane 1 versus 2). To control for TIEG2 NTD expression, transfected CHO cells were labeled with [35S]methionine and analyzed by anti-GAL4 immunoprecipitation as described above. Note that TIEG2 NTD is expressed at comparable levels. (C) GAL4-based reporter assays were performed in CHO cells transfected with GAL4 DBD alone or GAL4 NTD along with a GAL4 luciferase reporter construct. Note that TIEG2 NTD repression (0.12 ± 0.04 versus 0.79 ± 0.1 GAL4 DBD alone) is strongly antagonized by co-expression of caMEK1 (0.51 ± 0.1), caRas (0.51 ± 0.09) or vErbB (0.59 ± 0.1). The MEK1 inhibitor PD089059 reverses the inhibition of TIEG2 NTD by vErbB (0.20 ± 0.04). Also note that anisomycin, a JNK and p38 activator, slightly reduces the NTD-mediated repression (0.29 ± 0.05). (D) GST–CTD and GST alone were subjected to rERK2 phosphorylation and used in a gel-shift assay with a probe containing a GC-rich binding site for TIEG2. The specific complex that forms between the GST–CTD and probe is indicated on the right (asterisk). Note that the DNA-binding activity of TIEG2 CTD is unaffected by rERK2 treatment (lane 4 versus 5). GST alone does not bind the GC probe (lanes 2 and 3); lane 1 shows the mobility of the GC-rich probe in the absence of binding proteins.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.