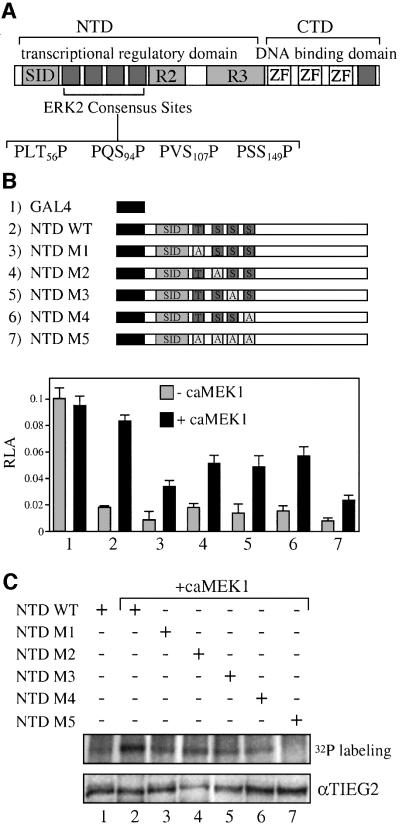
Fig. 4. ERK2 phosphorylation sites are adjacent to the TIEG2 SID. (A) A schematic representation of TIEG2 protein structure shows the three zinc finger motifs that bind DNA and repression domains SID, R2 and R3 within the NTD. Sequence analysis of the linker region found between SID and R2 reveals four putative MAP kinase phosphorylation sites (PLT56P, PQS94P, PVS107P and PSS149P). An additional site is present C-terminal to the zinc finger domains. (B) Alanine substitution mutants of the ERK2 sites were generated. GAL4 constructs encoding wild-type NTD (NTD WT), NTD with single point mutations (NTD M1–M4) or NTD with combined point mutations (NTD M5) were co-transfected into CHO cells along with the GAL4 reporter and caMEK1 as indicated. Note that co-transfection with caMEK1 antagonizes the repression activity of the wild-type NTD (lane 2) and to a lesser degree that of the GAL4 NTD mutants M1–M4 (lanes 3–6). The M5 mutant leads to the greatest reduction of MEK1-induced inhibition of the transcriptional repression activity. (C) As indicated, CHO cells were transfected with GAL4 NTD wild-type or mutant constructs along with caMEK1. Cells were then metabolically labeled with [32P]orthophosphate and anti-GAL4 immunoprecipitations were performed followed by audioradiography. Note that wild-type NTD phosphorylation is increased when caMEK1 is co-expressed (lanes 1 versus 2). CaMEK1-mediated phosphorylation of NTD M1–M4 (lanes 3, 4, 5 and 6, respectively) is reduced. NTD M5 abolishes caMEK1-induced phosphorylation (lane 7). Expression of the constructs was monitored by anti-TIEG2 western blots of cell lysates and shows that all constructs are expressed at comparable levels.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.