Abstract
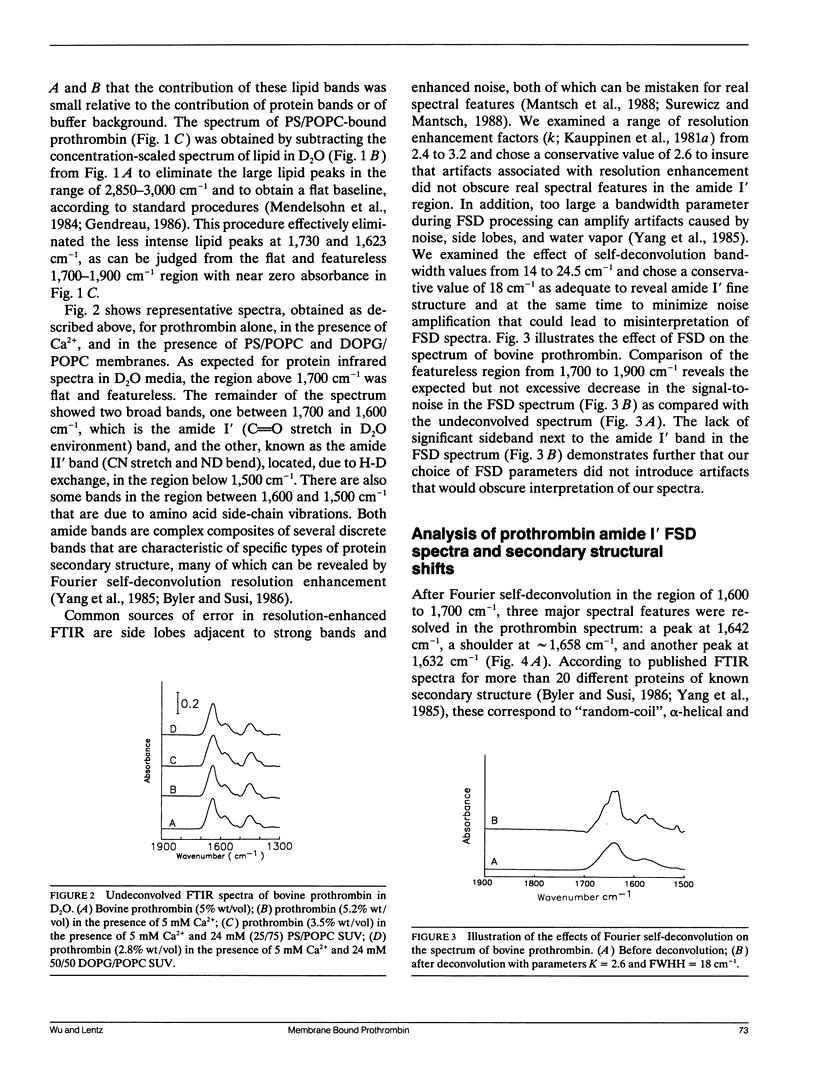
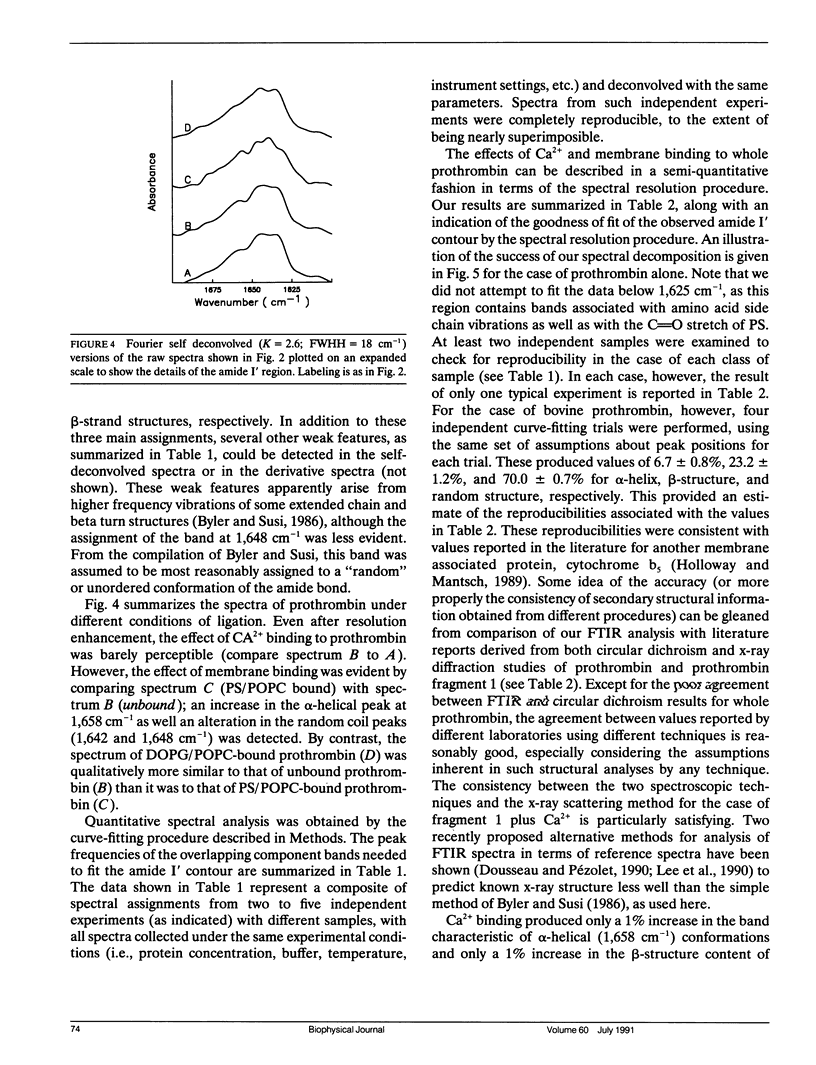
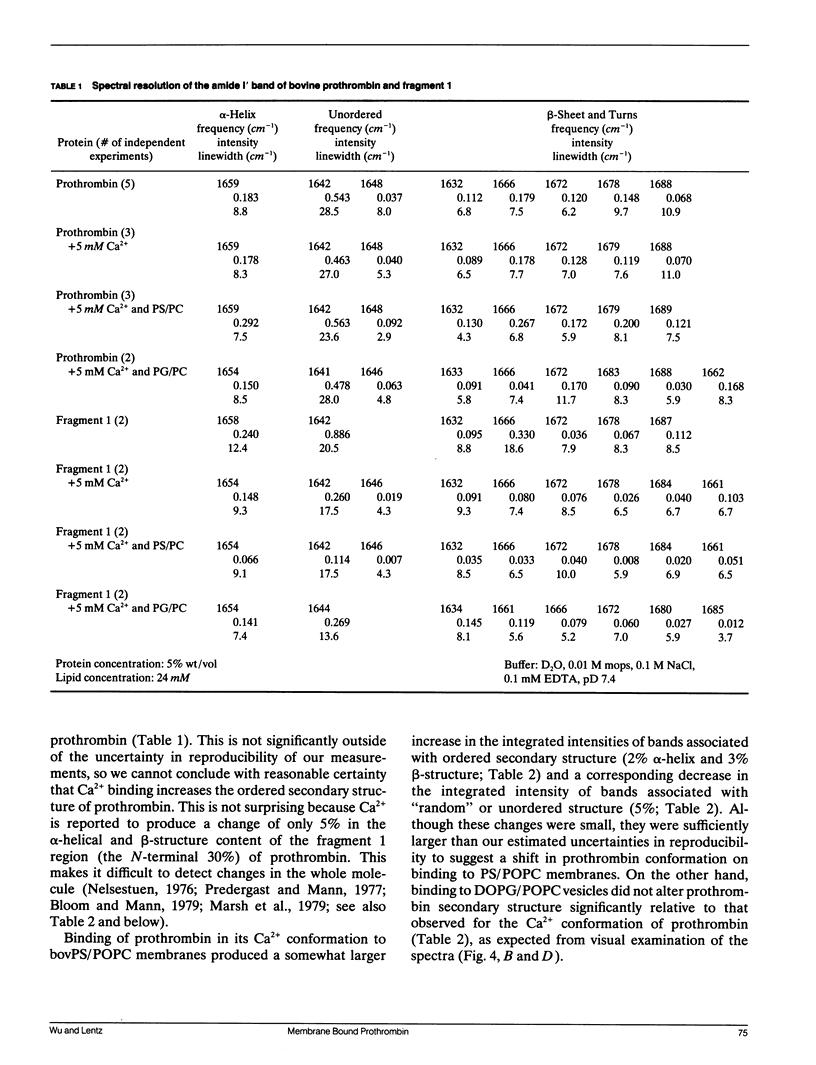
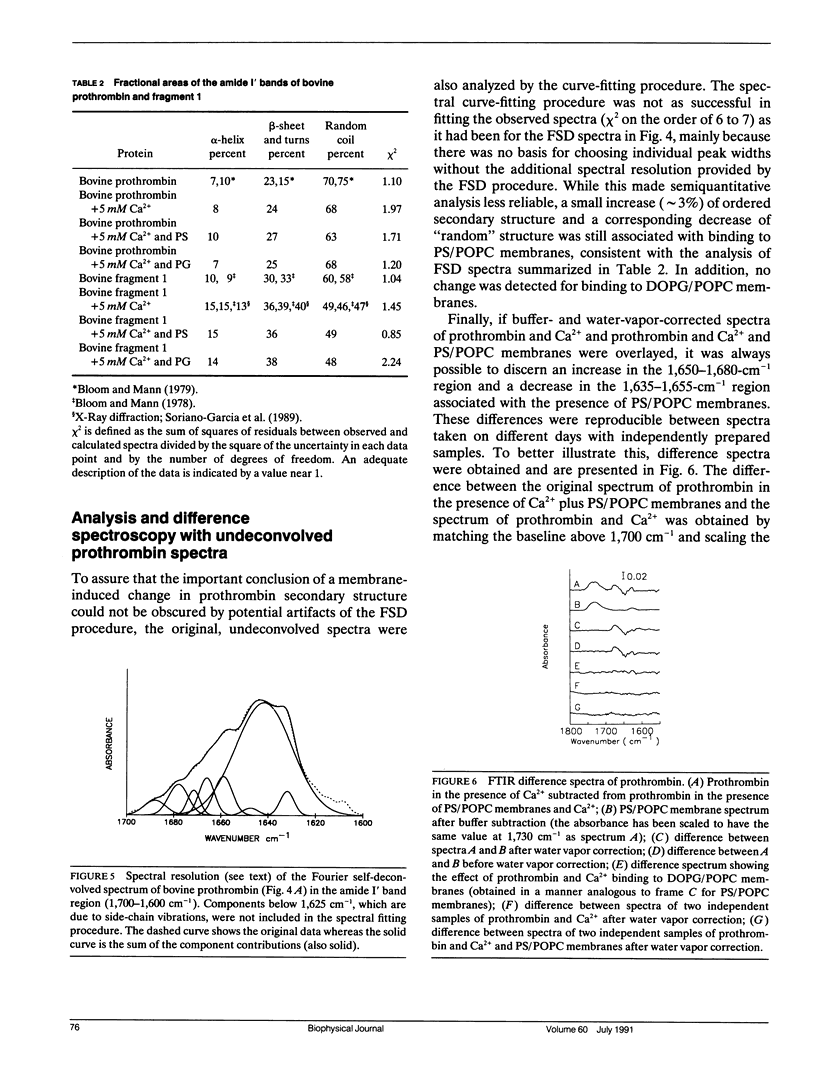
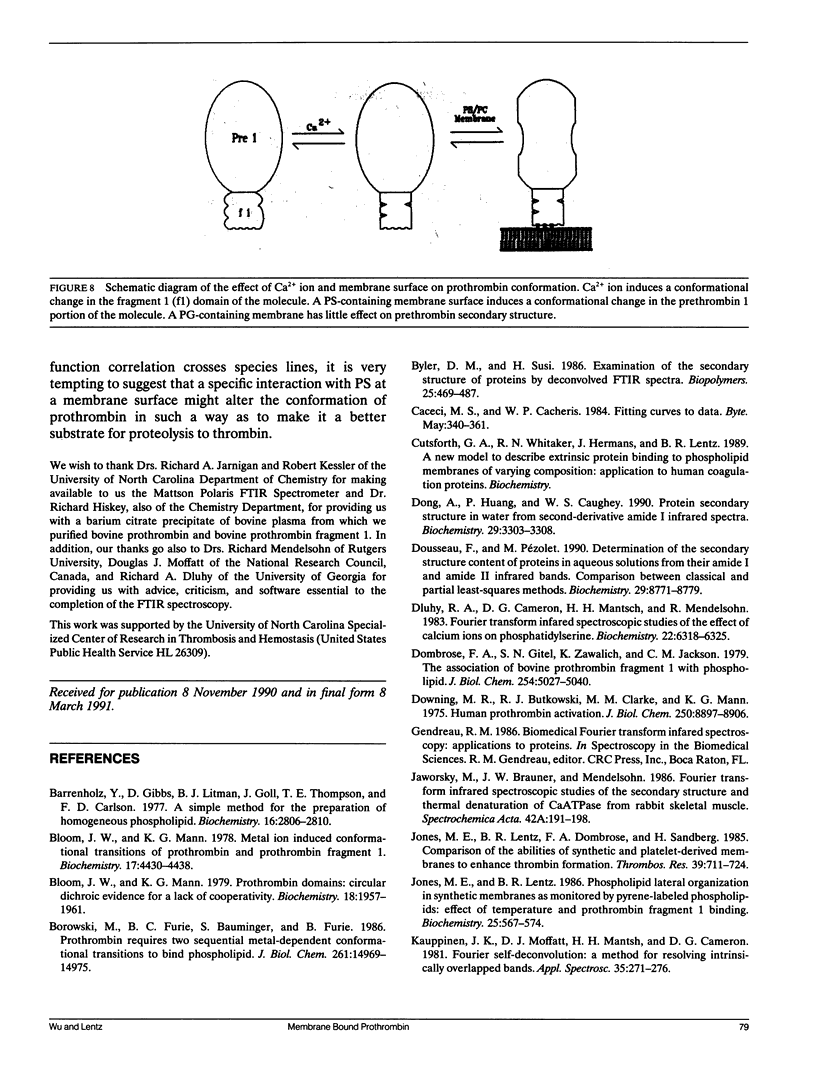
Fourier transform infrared (FTIR) spectroscopy was used to monitor secondary structural changes associated with binding of bovine prothrombin and prothrombin fragment 1 to acidic lipid membranes. Prothrombin and prothrombin fragment 1 were examined under four different conditions: in the presence of (a) Na2EDTA, (b) 5 mM CaCl2, and in the presence of CaCl2 plus membranes containing 1-palmitoyl-2-oleoyl-3-sn-phosphatidylcholine (POPC) in combination with either (c) bovine brain phosphatidyl-serine (bovPS) or (d) 1,2-dioleoyl-phosphatidylglycerol (DOPG). The widely reported Ca(2+)-induced conformational change in bovine prothrombin fragment 1 was properly detected by our procedures, although Ca(2+)-induced changes in whole prothrombin spectra were too small to be reliably interpreted. Binding of prothrombin in the presence of Ca2+ to procoagulant POPC/bovPS small unilamellar vesicles produced an increase in ordered secondary structures (2% and 3% increases in alpha-helix and beta-sheet, respectively) and a decrease of random structure (5%) as revealed by spectral analysis on both the original and Fourier-self-deconvolved data and by difference spectroscopy with the undeconvolved spectra. Binding to POPC/DOPG membranes, which are less active as procoagulant membranes, produced no detectable changes in secondary structure. In addition, no change in prothrombin fragment 1 secondary structure was detectable upon binding to either POPC/bovPS or POPC/DOPG membranes. This indicates that a membrane-induced conformational change occurs in prothrombin in the nonmembrane-binding portion of the molecule, part of which is activated to form thrombin, rather than in the membrane-binding fragment 1 region. The possible significance of this conformational change is discussed in terms of differences between the procoagulant activities of different acidic lipid membranes.
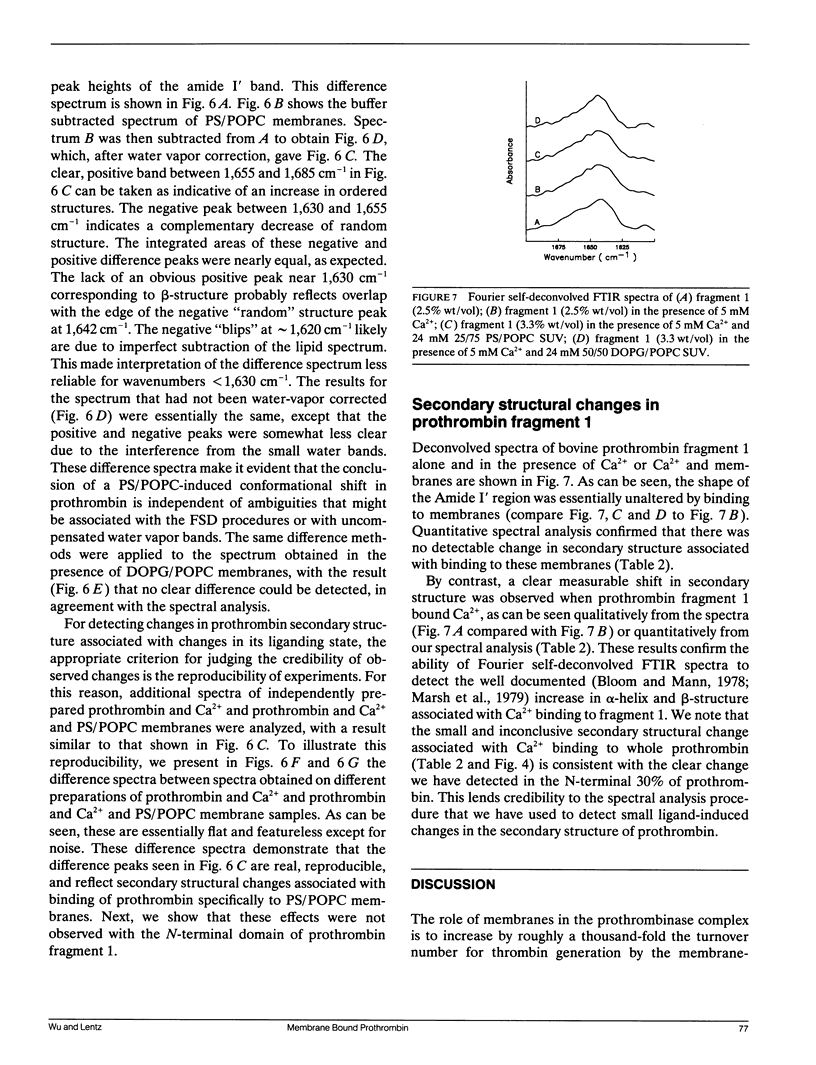
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenholz Y., Gibbes D., Litman B. J., Goll J., Thompson T. E., Carlson R. D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977 Jun 14;16(12):2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- Bloom J. W., Mann K. G. Metal ion induced conformational transitions of prothrombin and prothrombin fragment 1. Biochemistry. 1978 Oct 17;17(21):4430–4438. doi: 10.1021/bi00614a012. [DOI] [PubMed] [Google Scholar]
- Bloom J. W., Mann K. G. Prothrombin domains: circular dichroic evidence for a lack of cooperativity. Biochemistry. 1979 May 15;18(10):1957–1961. doi: 10.1021/bi00577a017. [DOI] [PubMed] [Google Scholar]
- Borowski M., Furie B. C., Bauminger S., Furie B. Prothrombin requires two sequential metal-dependent conformational transitions to bind phospholipid. Conformation-specific antibodies directed against the phospholipid-binding site on prothrombin. J Biol Chem. 1986 Nov 15;261(32):14969–14975. [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Dombrose F. A., Gitel S. N., Zawalich K., Jackson C. M. The association of bovine prothrombin fragment 1 with phospholipid. Quantitative characterization of the Ca2+ ion-mediated binding of prothrombin fragment 1 to phospholipid vesicles and a molecular model for its association with phospholipids. J Biol Chem. 1979 Jun 25;254(12):5027–5040. [PubMed] [Google Scholar]
- Dong A., Huang P., Caughey W. S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry. 1990 Apr 3;29(13):3303–3308. doi: 10.1021/bi00465a022. [DOI] [PubMed] [Google Scholar]
- Dousseau F., Pézolet M. Determination of the secondary structure content of proteins in aqueous solutions from their amide I and amide II infrared bands. Comparison between classical and partial least-squares methods. Biochemistry. 1990 Sep 18;29(37):8771–8779. doi: 10.1021/bi00489a038. [DOI] [PubMed] [Google Scholar]
- Downing M. R., Butkowski R. J., Clark M. M., Mann K. G. Human prothrombin activation. J Biol Chem. 1975 Dec 10;250(23):8897–8906. [PubMed] [Google Scholar]
- Jones M. E., Lentz B. R., Dombrose F. A., Sandberg H. Comparison of the abilities of synthetic and platelet-derived membranes to enhance thrombin formation. Thromb Res. 1985 Sep 15;39(6):711–724. doi: 10.1016/0049-3848(85)90255-5. [DOI] [PubMed] [Google Scholar]
- Jones M. E., Lentz B. R. Phospholipid lateral organization in synthetic membranes as monitored by pyrene-labeled phospholipids: effects of temperature and prothrombin fragment 1 binding. Biochemistry. 1986 Feb 11;25(3):567–574. doi: 10.1021/bi00351a009. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Haris P. I., Chapman D., Mitchell R. C. Determination of protein secondary structure using factor analysis of infrared spectra. Biochemistry. 1990 Oct 2;29(39):9185–9193. doi: 10.1021/bi00491a012. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Alford D. R., Jones M. E., Dombrose F. A. Calcium-dependent and calcium-independent interactions of prothrombin fragment 1 with phosphatidylglycerol/phosphatidylcholine unilamellar vesicles. Biochemistry. 1985 Nov 19;24(24):6997–7005. doi: 10.1021/bi00345a037. [DOI] [PubMed] [Google Scholar]
- Levitt M., Greer J. Automatic identification of secondary structure in globular proteins. J Mol Biol. 1977 Aug 5;114(2):181–239. doi: 10.1016/0022-2836(77)90207-8. [DOI] [PubMed] [Google Scholar]
- Lim T. K., Bloomfield V. A., Nelsestuen G. L. Structure of the prothrombin- and blood clotting factor X-membrane complexes. Biochemistry. 1977 Sep 20;16(19):4177–4181. doi: 10.1021/bi00638a007. [DOI] [PubMed] [Google Scholar]
- Mann K. G. Prothrombin. Methods Enzymol. 1976;45:123–156. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- Marsh H. C., Robertson P., Jr, Scott M. E., Koehler K. A., Hiskey R. G. Magnesium and calcium ion binding to bovine prothrombin fragment 1. A circular dichroism, fluorescence, and 43Ca2+ and 25Mg2+ nuclear magnetic resonance study. J Biol Chem. 1979 Oct 25;254(20):10268–10275. [PubMed] [Google Scholar]
- Mayer L. D., Nelsestuen G. L. Calcium and prothrombin-induced lateral phase separation in membranes. Biochemistry. 1981 Apr 28;20(9):2457–2463. doi: 10.1021/bi00512a015. [DOI] [PubMed] [Google Scholar]
- Mendelsohn R., Anderle G., Jaworsky M., Mantsch H. H., Dluhy R. A. Fourier transform infrared spectroscopic studies of lipid-protein interaction in native and reconstituted sarcoplasmic reticulum. Biochim Biophys Acta. 1984 Aug 22;775(2):215–224. doi: 10.1016/0005-2736(84)90173-1. [DOI] [PubMed] [Google Scholar]
- Nelsestuen G. L., Broderius M., Martin G. Role of gamma-carboxyglutamic acid. Cation specificity of prothrombin and factor X-phospholipid binding. J Biol Chem. 1976 Nov 25;251(22):6886–6893. [PubMed] [Google Scholar]
- Nelsestuen G. L. Role of gamma-carboxyglutamic acid. An unusual protein transition required for the calcium-dependent binding of prothrombin to phospholipid. J Biol Chem. 1976 Sep 25;251(18):5648–5656. [PubMed] [Google Scholar]
- Ploplis V. A., Strickland D. K., Castellino F. J. Calorimetric evaluation of the existence of separate domains in bovine prothrombin. Biochemistry. 1981 Jan 6;20(1):15–21. doi: 10.1021/bi00504a003. [DOI] [PubMed] [Google Scholar]
- Prendergast F. G., Mann K. G. Differentiation of metal ion-induced transitions of prothrombin fragment 1. J Biol Chem. 1977 Feb 10;252(3):840–850. [PubMed] [Google Scholar]
- Rosing J., Speijer H., Zwaal R. F. Prothrombin activation on phospholipid membranes with positive electrostatic potential. Biochemistry. 1988 Jan 12;27(1):8–11. doi: 10.1021/bi00401a002. [DOI] [PubMed] [Google Scholar]
- Rosing J., Tans G., Govers-Riemslag J. W., Zwaal R. F., Hemker H. C. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980 Jan 10;255(1):274–283. [PubMed] [Google Scholar]
- Soriano-Garcia M., Park C. H., Tulinsky A., Ravichandran K. G., Skrzypczak-Jankun E. Structure of Ca2+ prothrombin fragment 1 including the conformation of the Gla domain. Biochemistry. 1989 Aug 22;28(17):6805–6810. doi: 10.1021/bi00443a004. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta. 1988 Jan 29;952(2):115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Tendian S. W., Lentz B. R. Evaluation of membrane phase behavior as a tool to detect extrinsic protein-induced domain formation: binding of prothrombin to phosphatidylserine/phosphatidylcholine vesicles. Biochemistry. 1990 Jul 17;29(28):6720–6729. doi: 10.1021/bi00480a023. [DOI] [PubMed] [Google Scholar]
- Wei G. J., Bloomfield V. A., Resnick R. M., Nelsestuen G. L. Kinetic and mechanistic analysis of prothrombin-membrane binding by stopped-flow light scattering. Biochemistry. 1982 Apr 13;21(8):1949–1959. doi: 10.1021/bi00537a039. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Hemker H. C. Blood cell membranes and haemostasis. Haemostasis. 1982;11(1):12–39. doi: 10.1159/000214638. [DOI] [PubMed] [Google Scholar]


