Abstract
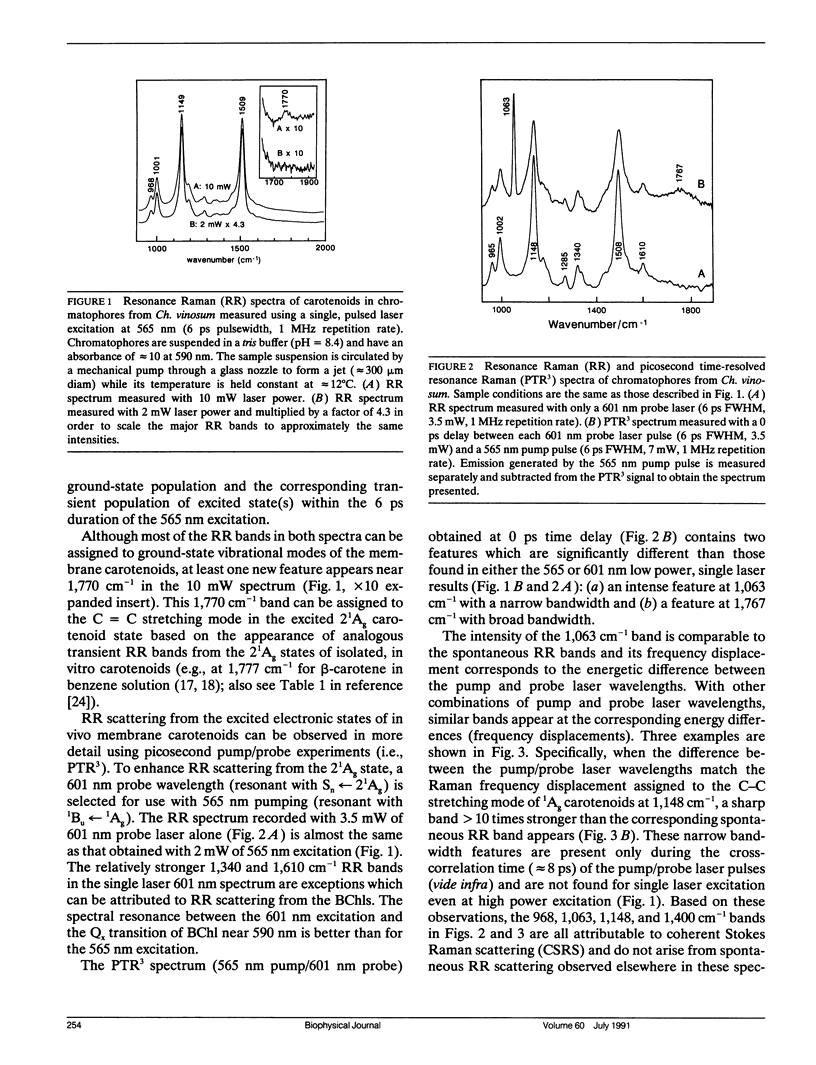
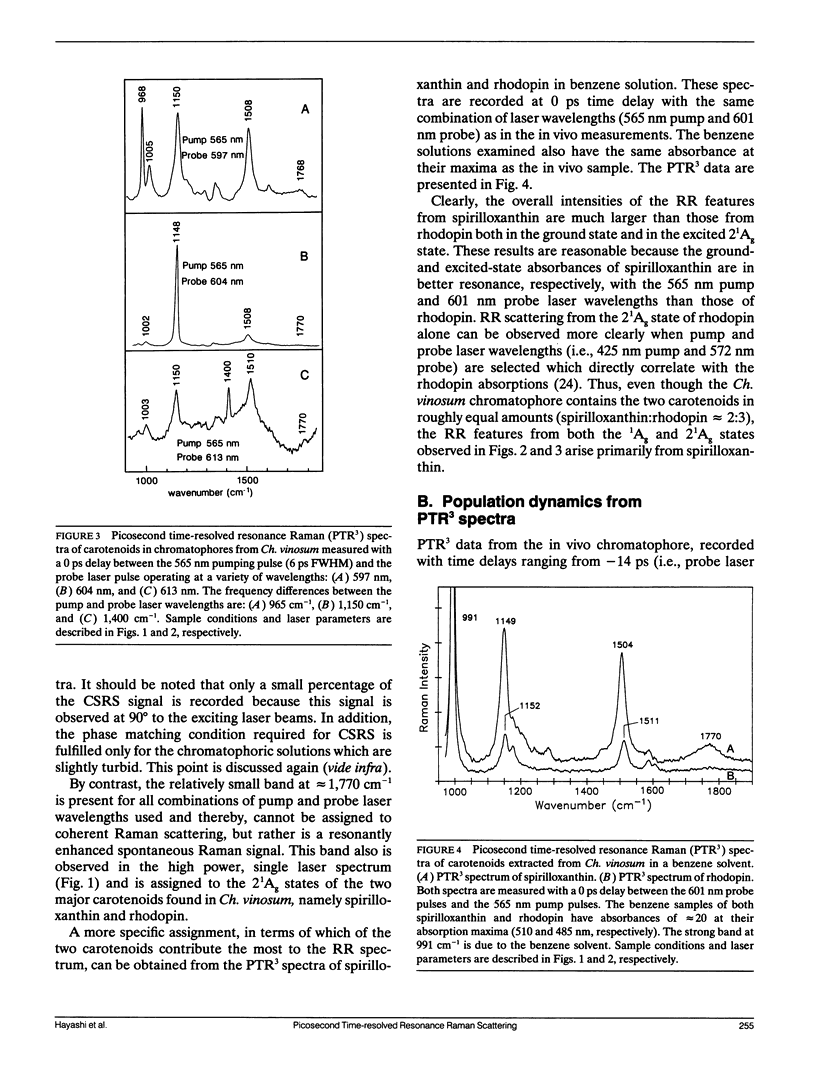
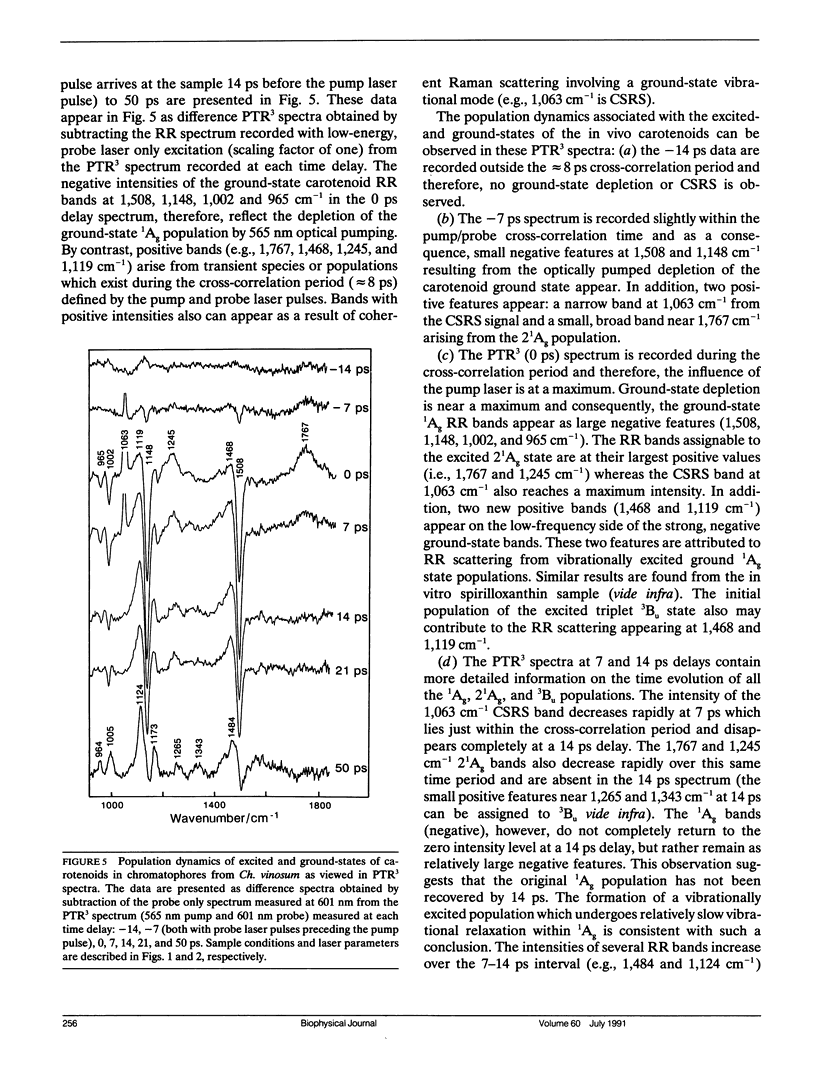
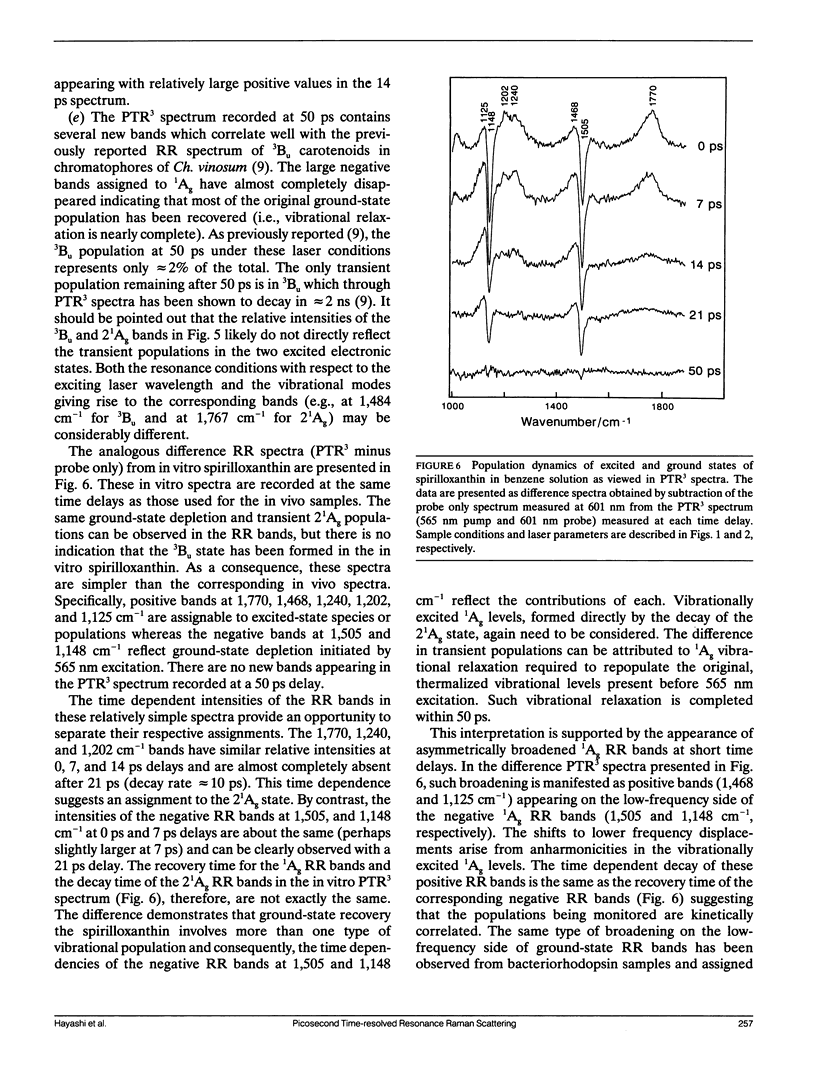
The vibrational spectroscopy and population dynamics of excited singlet (2(1)Ag), excited triplet (3B u), and the ground (1Ag) electronic states of carotenoids in chromatophores of Chromatium vinosum (mainly spirilloxanthin and rhodopin) and of the same carotenoids in benzene solutions are examined by picosecond time-resolved resonance Raman scattering. Coherent Stokes Raman scattering from the ground states of carotenoids in chromatophores also is observed. Resonance Raman spectra of in vitro rhodopin and spirilloxanthin when compared with in vivo data demonstrate that scattering from spirilloxanthin dominates the in vivo spectrum. Comparisons of the time-dependent intensities of 2(1)Ag and 1Ag resonance Raman bands from both in vitro and in vivo carotenoids suggest that vibrationally excited levels in 1Ag are populated directly by the decay of the 2(1)Ag state and that these levels relax into a thermalized distribution in less than 50 ps. The appearance of asymmetrically broadened, ground-state resonance Raman bands supports this conclusion. Formation of the 3Bu state is observed for carotenoids in chromatophores, but not for in vitro spirilloxanthin indicating that the 3Bu state is formed by fission processes originating from the spatial organization of pigments within chromatophores. The rate at which the intensities of 2(1)Ag resonance Raman bands decay is faster for the carotenoids in vivo than for those in vitro thereby indicating that additional relaxation channels (e.g., energy transfer to bacteriochlorophylls) are present in the chromatophore. The similarity of the in vivo and in vitro 2(1)Ag resonance Raman spectra shows that no significant modifications in the vibronic coupling has been caused by the chromatophore environment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson G. H., Blanchard D., Lemaire H., Brack T. L., Hayashi H. Picosecond time-resolved fluorescence spectroscopy of K-590 in the bacteriorhodopsin photocycle. Biophys J. 1989 Feb;55(2):263–274. doi: 10.1016/S0006-3495(89)82801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell R. J., Frank H. A. How carotenoids function in photosynthetic bacteria. Biochim Biophys Acta. 1987;895(2):63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- Frank H. A., McGann W. J., Macknicki J., Felber M. Magnetic field effects on the fluorescence of two reaction centerless mutants of Rhodopseudomonas capsulata. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1310–1317. doi: 10.1016/0006-291x(82)91256-6. [DOI] [PubMed] [Google Scholar]
- Rademaker H., Hoff A. J., van Grondelle R., Duysens L. N. Carotenoid triplet yields in normal and deuterated Rhodospirillum rubrum. Biochim Biophys Acta. 1980 Sep 5;592(2):240–257. doi: 10.1016/0005-2728(80)90185-1. [DOI] [PubMed] [Google Scholar]
- Trautman J. K., Shreve A. P., Violette C. A., Frank H. A., Owens T. G., Albrecht A. C. Femtosecond dynamics of energy transfer in B800-850 light-harvesting complexes of Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Jan;87(1):215–219. doi: 10.1073/pnas.87.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]


