Abstract
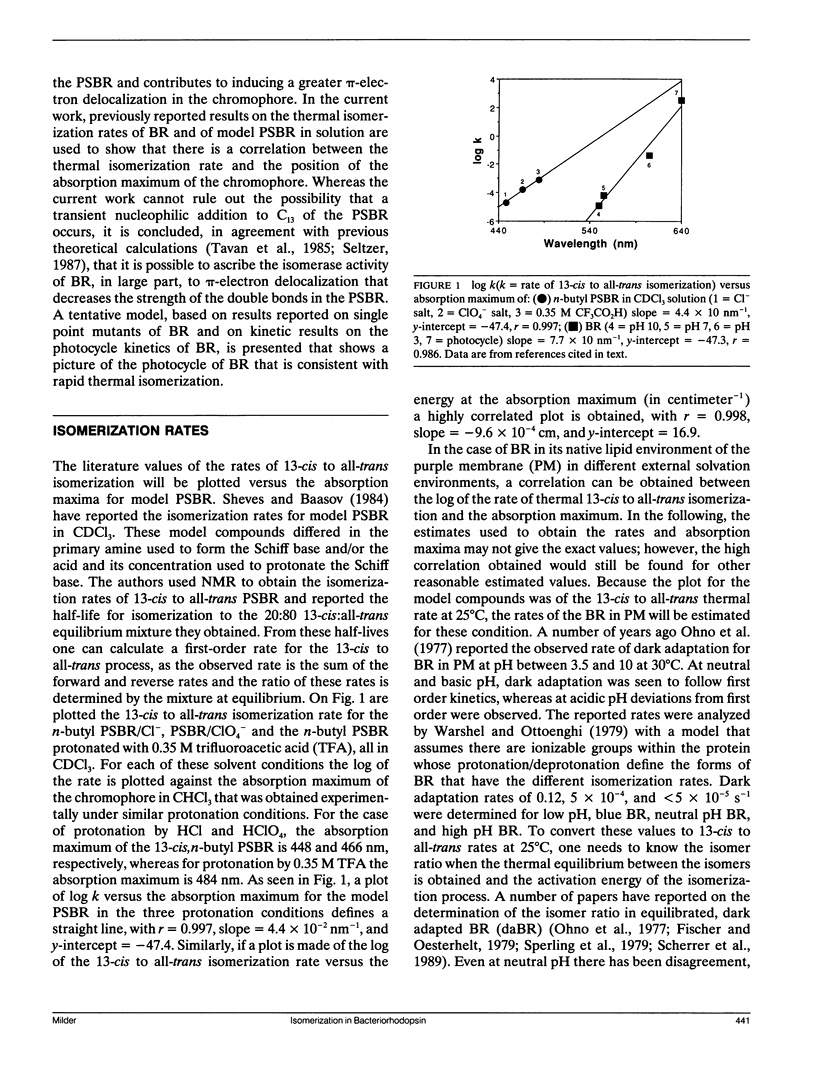
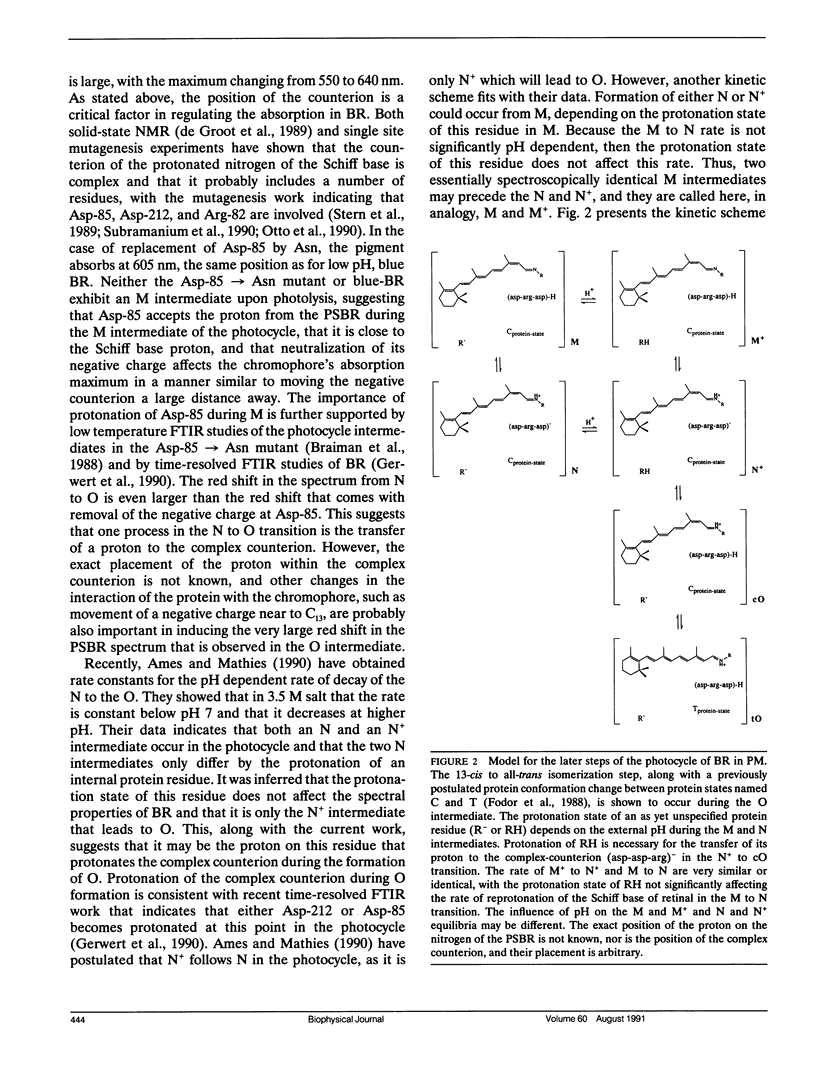
The reported rates of thermal 13-cis to all-trans isomerization of the protonated Schiff base of retinal (PSBR) in solution and in bacteriorhodopsin (BR) are shown to be correlated with the red shift in the absorption maximum of the chromophore, though the linear fit is different for BR and for a model PSBR in solution. Because the red shift in the absorption has been previously shown to be correlated with π-electron delocalization in the chromophore, this suggests that the thermal isomerization rate is largely regulated by the amount of double bond character in the chromophore. Because the linear fit of isomerization rates with absorption maxima is different for BR and the model PSBR, specific interactions of the protein with the chromophore must also be a factor in determining thermal isomerization rates in BR. A model of the later steps in the photocycle of BR is presented in which the 13-cis to all-trans thermal isomerization occurs during the O intermediate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Fodor S. P., Gebhard R., Raap J., van den Berg E. M., Lugtenburg J., Mathies R. A. Bacteriorhodopsin's M412 intermediate contains a 13-cis, 14-s-trans, 15-anti-retinal Schiff base chromophore. Biochemistry. 1989 May 2;28(9):3681–3687. doi: 10.1021/bi00435a009. [DOI] [PubMed] [Google Scholar]
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas A. G., Aton B., Callender R. H., Ebrey T. G. Resonance Raman studies of bovine metarhodopsin I and metarhodopsin II. Biochemistry. 1978 Jun 13;17(12):2430–2435. doi: 10.1021/bi00605a028. [DOI] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Gerwert K., Souvignier G., Hess B. Simultaneous monitoring of light-induced changes in protein side-group protonation, chromophore isomerization, and backbone motion of bacteriorhodopsin by time-resolved Fourier-transform infrared spectroscopy. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9774–9778. doi: 10.1073/pnas.87.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Lozier R. H. Photocycles of bacteriorhodopsin in light- and dark-adapted purple membrane studied by time-resolved absorption spectroscopy. Biophys J. 1989 Oct;56(4):693–706. doi: 10.1016/S0006-3495(89)82716-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas R., Ebrey T. G. Binding of a single divalent cation directly correlates with the blue-to-purple transition in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):149–153. doi: 10.1073/pnas.88.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Resonance Raman spectroscopy of the retinylidene chromophore in bacteriorhodopsin (bR570), bR560, M421, and other intermediates: structural conclusions based on kinetics, analogues, models, and isotopically labeled membranes. Biochemistry. 1978 Oct 31;17(22):4722–4735. doi: 10.1021/bi00615a019. [DOI] [PubMed] [Google Scholar]
- Milder S. J., Thorgeirsson T. E., Miercke L. J., Stroud R. M., Kliger D. S. Effects of detergent environments on the photocycle of purified monomeric bacteriorhodopsin. Biochemistry. 1991 Feb 19;30(7):1751–1761. doi: 10.1021/bi00221a004. [DOI] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretations of the effects of pH on the spectra of purple membrane. J Mol Biol. 1979 Dec 15;135(3):595–609. doi: 10.1016/0022-2836(79)90166-9. [DOI] [PubMed] [Google Scholar]
- Ohno K., Takeuchi Y., Yoshida M. Effect of light-adaptation on the photoreaction of bacteriorhodopsin from Halobacterium halobium. Biochim Biophys Acta. 1977 Dec 23;462(3):575–582. doi: 10.1016/0005-2728(77)90102-5. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi M., Sheves M. Synthetic retinals as probes for the binding site and photoreactions in rhodopsins. J Membr Biol. 1989 Dec;112(3):193–212. doi: 10.1007/BF01870951. [DOI] [PubMed] [Google Scholar]
- Rimai L., Heyde M. E., Gill D. Vibrational spectra of some carotenoids and related linear polyenes. A Raman spectroscopic study. J Am Chem Soc. 1973 Jul 11;95(14):4493–4501. doi: 10.1021/ja00795a005. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Marrero H. Infrared evidence that the Schiff base of bacteriorhodopsin is protonated: bR570 and K intermediates. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4045–4049. doi: 10.1073/pnas.79.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer P., Mathew M. K., Sperling W., Stoeckenius W. Retinal isomer ratio in dark-adapted purple membrane and bacteriorhodopsin monomers. Biochemistry. 1989 Jan 24;28(2):829–834. doi: 10.1021/bi00428a063. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Palings I., Miley M. E., Courtin J., de Groot H., Lugtenburg J., Mathies R. A., Griffin R. G. Solid-state NMR studies of the mechanism of the opsin shift in the visual pigment rhodopsin. Biochemistry. 1990 Sep 4;29(35):8158–8164. doi: 10.1021/bi00487a025. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Ahl P. L., Marti T., Mogi T., Duñach M., Berkowitz S., Rothschild K. J., Khorana H. G. Substitution of membrane-embedded aspartic acids in bacteriorhodopsin causes specific changes in different steps of the photochemical cycle. Biochemistry. 1989 Dec 26;28(26):10035–10042. doi: 10.1021/bi00452a023. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Khorana H. G. Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82----Ala and Asp-85----Glu: the blue form is inactive in proton translocation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1013–1017. doi: 10.1073/pnas.87.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavan P., Schulten K., Oesterhelt D. The effect of protonation and electrical interactions on the stereochemistry of retinal schiff bases. Biophys J. 1985 Mar;47(3):415–430. doi: 10.1016/S0006-3495(85)83933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Pathways of the rise and decay of the M photointermediate(s) of bacteriorhodopsin. Biochemistry. 1990 Mar 6;29(9):2241–2250. doi: 10.1021/bi00461a006. [DOI] [PubMed] [Google Scholar]
- Warshel A. Charge stabilization mechanism in the visual and purple membrane pigments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2558–2562. doi: 10.1073/pnas.75.6.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Ottolenighi M. Kinetic and spectroscopic effects of protein-chromophore electrostatic interactions in bacteriorhodopsin. Photochem Photobiol. 1979 Aug;30(2):291–293. doi: 10.1111/j.1751-1097.1979.tb07149.x. [DOI] [PubMed] [Google Scholar]
- de Groot H. J., Harbison G. S., Herzfeld J., Griffin R. G. Nuclear magnetic resonance study of the Schiff base in bacteriorhodopsin: counterion effects on the 15N shift anisotropy. Biochemistry. 1989 Apr 18;28(8):3346–3353. doi: 10.1021/bi00434a033. [DOI] [PubMed] [Google Scholar]


