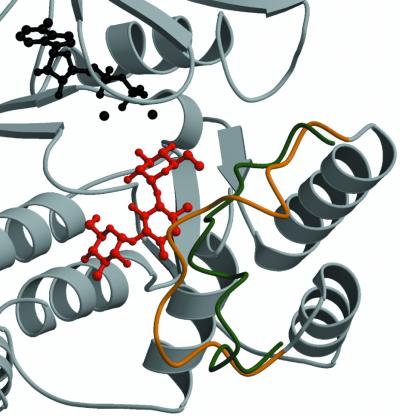
Fig. 3. Structural changes observed in APH(3′)-IIIa upon aminoglycoside binding. Shown is a ribbon diagram of the APH(3′)-IIIa– kanamycin A ternary complex in the vicinity of the antibiotic binding pocket. The Cα trace of the aminoglycoside-binding loop (residues 150–165) is in gold, the remainder of the protein is in gray, the antibiotic is red, and the ADP co-factor and the magnesium ions are displayed in black. Overlaid is a backbone trace for residues 150–165 of the APH(3′)-IIIa–ADP holoenzyme structure (Burk et al., 2001), colored in green. The aminoglycoside-binding loop represents the largest conformational difference between ternary complexes and holoenzyme structures.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.