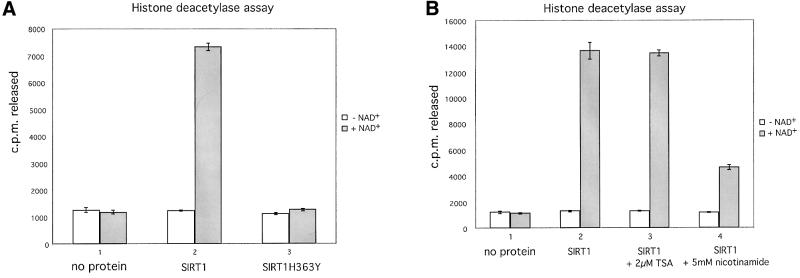
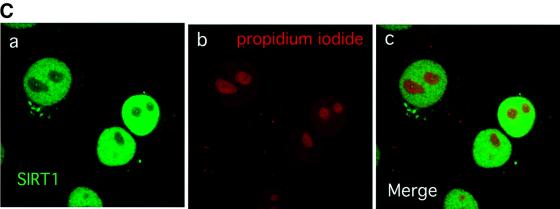
Fig. 1. SIRT1 is an active nuclear NAD-dependent HDAC. (A) Equivalent amounts of wild-type His-SIRT1 or mutant His-SIRT1H363Y were tested for HDAC activity in the presence (full column) or absence (open column) of 1 mM NAD+. Histone deacetylase activity is given as radioactivity (c.p.m.) of [3H]acetate released from an acetylated histone H4 peptide. Control assays containing only acetylated H4 peptide without any recombinant protein were performed. (B) NAD-dependent HDAC assay performed essentially as above in which GST–SIRT1 was tested for activity in the absence (2) or presence of either 2 µM TSA (3) or 5 mM nicotinamide (4). All reactions contain identical amounts of GST–SIRT1. (C) Asynchronous HeLa cells were analyzed for endogenous SIRT1 expression using an anti-SIRT1 antibody followed by incubation with an Alexa 488-conjugated secondary antibody (green). Nuclear DNA was stained with propidium iodide (red). Note the high level of propidium iodide staining in the nucleoli.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.