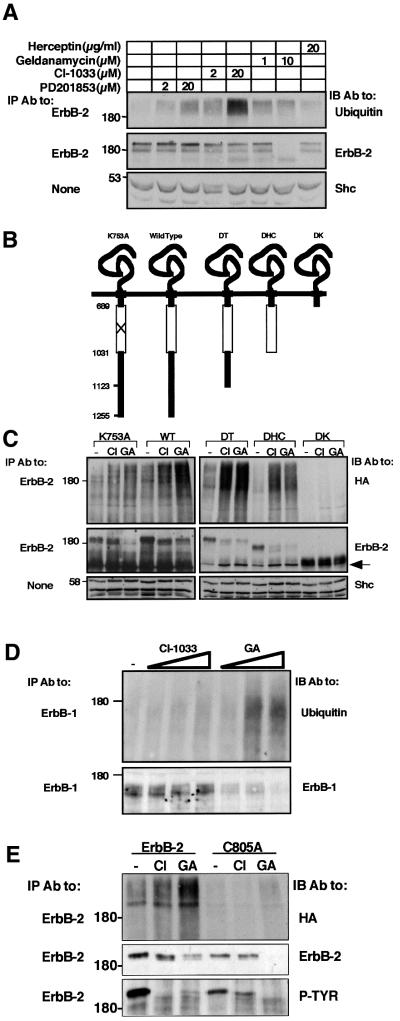
Fig. 4. Receptor sensitivity to drug-induced ubiquitylation and degradation is conferred by the kinase domain. (A) Liquid cultures of D2 cells were spun and resuspended in medium containing the indicated agents. Following 2 h of incubation at 37°C, cells were re-spun and their lysates subjected to IP and IB with the indicated antibodies. For control, whole-cell extracts were subjected to IB with antibodies to Shc. (B) Schematic representation of ErbB-2 mutant proteins. The horizontal line represents the plasma membrane and numbers indicate the amino acid sites of truncations. The open box marks the tyrosine kinase domain. (C) The indicated ErbB-2 constructs were expressed in CHO cells along with HA-Ub. Forty-eight hours after transfection, cells were treated with CI-1033 (CI; 5 µM), GA (1 µM) or DMSO (lanes labeled –) for 90 min. Cell extracts were prepared and analyzed with the indicated antibodies. IB for ErbB-2 was carried out using an antibody directed to the extracellular domain. An arrow marks a 135 kDa degradation product of ErbB-2. (D) 32D cells stably expressing a kinase-defective ErbB-1 mutant were treated for 3 h with increasing concentrations of CI-1033 (0.2, 2 or 20 µM) or GA (0.1, 1 or 10 µM). Thereafter, cell extracts were prepared and the ErbB-1 protein analyzed with the indicated antibodies. (E) HEK-293T cells were transfected with a plasmid encoding HA-Ub, along with expression vectors for either wild-type ErbB-2 or a mutant at Cys805 (C805A). Eighteen hours later, cells were treated for 20 min with CI-1033 (10 µM) or GA (1 µM), or left untreated for control. Thereafter, cells were washed and incubated for 4 h in fresh drug-free media prior to analysis of ErbB-2 with the indicated antibodies.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.