Abstract
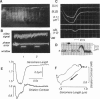
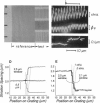
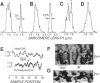
We describe an extension of the method of Myers et al. (1982) to measure with high precision the uniformity of contractile motions that occur between sarcomeres in the isolated cardiac muscle cell (guinea pig and rat). The image of the striations, observed with modulation contrast microscopy, was detected by a linear array of photodiodes. Sarcomere length was measured greater than 500/s from the frequency of the array's video signal at two selectable regions of the cell. A precision test grating demonstrated that method resolves known differences in the spacing between two contiguous striations to +/- 0.01 micron and that the effects of image translation and microscopic resolution are minor. The distribution of striation spacing appears to be discrete in isolated segments of the cell, and patches of fairly uniform length can be identified that are laterally contiguous. When electrically triggered, contraction is synchronous and the sarcomeres shorten and relengthen smoothly. The contrast between the striations is transiently enhanced during relengthening, an indication that the contracting cell can not be treated as a simple grating. Pauses that occur during late in relengthening (and transient contractile alternans) are characterized by very synchronized activity. These forms of irregular contractile behavior are not explained by desynchronization of a mechanism of release of intracellular calcium. A companion article describes application of the technique to study the nonuniform motions that occur between sarcomeres.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altringham J. D., Bottinelli R., Lacktis J. W. Is stepwise sarcomere shortening an artefact? Nature. 1984 Feb 16;307(5952):653–655. doi: 10.1038/307653a0. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Actions of adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1983 Sep;53(3):287–297. doi: 10.1161/01.res.53.3.287. [DOI] [PubMed] [Google Scholar]
- Berlin J. R., Cannell M. B., Lederer W. J. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res. 1989 Jul;65(1):115–126. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Lakatta E. G. Frequency modulation and synchronization of spontaneous oscillations in cardiac cells. Am J Physiol. 1985 Mar;248(3 Pt 2):H412–H418. doi: 10.1152/ajpheart.1985.248.3.H412. [DOI] [PubMed] [Google Scholar]
- De Clerck N. M., Claes V. A., Brutsaert D. L. Uniform sarcomere behaviour during twitch of intact single cardiac cells. J Mol Cell Cardiol. 1984 Aug;16(8):735–745. doi: 10.1016/s0022-2828(84)80657-4. [DOI] [PubMed] [Google Scholar]
- De Clerck N. M., Claes V. A., Van Ocken E. R., Brutsaert D. L. Sarcomere distribution patterns in single cardiac cells. Biophys J. 1981 Jul;35(1):237–242. doi: 10.1016/S0006-3495(81)84784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Challice C. E., Guilbault P., Dupuis B. Two components of contraction in guinea pig papillary muscle. Can J Physiol Pharmacol. 1986 Sep;64(9):1153–1159. doi: 10.1139/y86-196. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Ca entry and contraction as studied in isolated bovine ventricular myocytes. Z Naturforsch C. 1982 May-Jun;37(5-6):502–512. doi: 10.1515/znc-1982-5-623. [DOI] [PubMed] [Google Scholar]
- Kort A. A., Capogrossi M. C., Lakatta E. G. Frequency, amplitude, and propagation velocity of spontaneous Ca++-dependent contractile waves in intact adult rat cardiac muscle and isolated myocytes. Circ Res. 1985 Dec;57(6):844–855. doi: 10.1161/01.res.57.6.844. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Denton A., Siciliano G. Nature of motions between sarcomeres in asynchronously contracting cardiac muscle cells. Biophys J. 1992 Jan;61(1):145–160. doi: 10.1016/S0006-3495(92)81823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. W., Forletti D., Wittenberg B. A. Uniform sarcomere shortening behavior in isolated cardiac muscle cells. J Gen Physiol. 1980 Nov;76(5):587–607. doi: 10.1085/jgp.76.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. W., London B. Contraction bands: differences between physiologically vs. maximally activated single heart muscle cells. Adv Exp Med Biol. 1984;170:119–134. doi: 10.1007/978-1-4684-4703-3_11. [DOI] [PubMed] [Google Scholar]
- Leung A. F. Sarcomere dynamics in single myocardial cells as revealed by high-resolution light diffractometry. J Muscle Res Cell Motil. 1983 Aug;4(4):485–502. doi: 10.1007/BF00711951. [DOI] [PubMed] [Google Scholar]
- London B., Krueger J. W. Contraction in voltage-clamped, internally perfused single heart cells. J Gen Physiol. 1986 Oct;88(4):475–505. doi: 10.1085/jgp.88.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad A., Gonzalez-Serratos H., Inesi G., Swanson J., Paolini P. Patterns of sarcomere activation, temperature dependence, and effect of ryanodine in chemically skinned cardiac fibers. J Gen Physiol. 1986 Jun;87(6):885–905. doi: 10.1085/jgp.87.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Tirosh R., Jacobson R. C., Pollack G. H. Phase-locked loop measurement of sarcomere length with high time resolution. IEEE Trans Biomed Eng. 1982 Jun;29(6):463–466. doi: 10.1109/TBME.1982.324975. [DOI] [PubMed] [Google Scholar]
- Pollack G. H. Is stepwise sarcomere shortening an artefact? A response. Nature. 1984 Jun 21;309(5970):712–714. doi: 10.1038/309712a0. [DOI] [PubMed] [Google Scholar]
- Pollack G. H. Quantal mechanisms in cardiac contraction. Circ Res. 1986 Jul;59(1):1–8. doi: 10.1161/01.res.59.1.1. [DOI] [PubMed] [Google Scholar]
- Rieser G., Sabbadini R., Paolini P., Fry M., Inesi G. Sarcomere motion in isolated cardiac cells. Am J Physiol. 1979 Jan;236(1):C70–C77. doi: 10.1152/ajpcell.1979.236.1.C70. [DOI] [PubMed] [Google Scholar]
- Roos K. P., Brady A. J. Individual sarcomere length determination from isolated cardiac cells using high-resolution optical microscopy and digital image processing. Biophys J. 1982 Dec;40(3):233–244. doi: 10.1016/S0006-3495(82)84478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos K. P., Brady A. J. Osmotic compression and stiffness changes in relaxed skinned cardiac myocytes in PVP-40 and dextran T-500. Biophys J. 1990 Nov;58(5):1273–1283. doi: 10.1016/S0006-3495(90)82467-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos K. P. Sarcomere length uniformity determined from three-dimensional reconstructions of resting isolated heart cell striation patterns. Biophys J. 1987 Aug;52(2):317–327. doi: 10.1016/S0006-3495(87)83219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Gelles J., Sheetz M. P. Nanometer-scale measurements using video light microscopy. Cell Motil Cytoskeleton. 1988;10(1-2):47–53. doi: 10.1002/cm.970100109. [DOI] [PubMed] [Google Scholar]
- Tameyasu T., Toyoki T., Sugi H. Nonsteady motion in unloaded contractions of single frog cardiac cells. Biophys J. 1985 Sep;48(3):461–465. doi: 10.1016/S0006-3495(85)83801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]