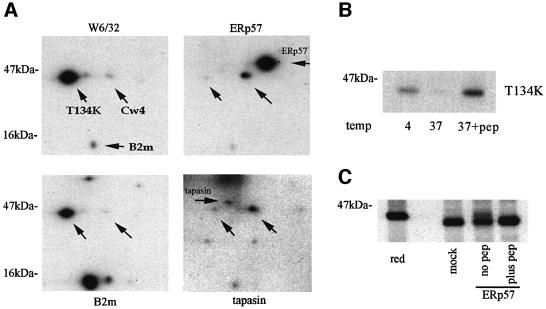
Fig. 5. T134K mutant A2 molecules form weak interactions with ERp57 and tapasin, but display similar resistance to ERp57 reduction. (A) Two- dimensional gel analysis of T134K immunoprecipitations. C1R-T134K cells were radiolabelled and immunoprecipitated with W6/32, anti-human B2m, anti-ERp57 and anti-tapasin. Upward pointing arrows indicated the positions of T134K molecules and the endogenous HLA-Cw4 molecule. Horizontal arrows indicate the position of B2m, ERp57 and tapasin as indicated. T134K is the major assembled MHC class I species in these cells (left panels). A short pre-clearing step results in the detection of T134K/B2m complexes that dissociate under longer incubation times. In comparison with Cw4, T134K forms a weak interaction with ERp57 and tapasin (right panels). Short labelling times result in the detection of the MHC class I molecules and the relevant chaperone being immunoprecipitated, and not the full peptide-loading complex. (B) Peptide stabilization of T134K molecules. Lysates of radiolabelled C1R-T134K cells were incubated at 4°C, or at 37°C in the presence or absence of 50 µM A2 binding peptide LLDVPTAAV, followed by immunoprecipitation with BB7.2. (C) BB7.2 immunoprecipitates from radiolabelled C1R-T134K lysates, and a peptide stabilized population (plus pep) were incubated with 1 µM ERp57, or mock-treated with assay buffer alone. The arrow indicates a minor population of reduced T134K heavy chains detected in the non-peptide stabilized sample (no pep).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.